Formulation information for generic ingredient: amlodipine besylate; atorvastatin calcium
✉ Email this page to a colleague
AMLODIPINE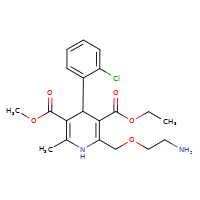
CASRN: 88150-42-9
Manufacturing Methods
…<truncated in preview>
ATORVASTATIN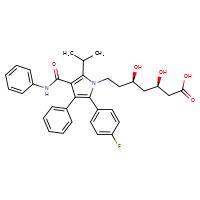
CASRN: 134523-00-5
Manufacturing Methods
…<truncated in preview>


