Viiv Hlthcare Company Profile
✉ Email this page to a colleague
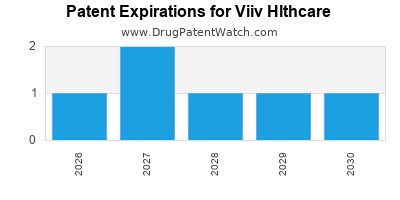
What is the competitive landscape for VIIV HLTHCARE, and when can generic versions of VIIV HLTHCARE drugs launch?
VIIV HLTHCARE has twenty-six approved drugs.
There are twelve US patents protecting VIIV HLTHCARE drugs.
There are five hundred and fourteen patent family members on VIIV HLTHCARE drugs in fifty-nine countries and two hundred and eleven supplementary protection certificates in eighteen countries.
Summary for Viiv Hlthcare
| International Patents: | 514 |
| US Patents: | 12 |
| Tradenames: | 19 |
| Ingredients: | 17 |
| NDAs: | 26 |
Drugs and US Patents for Viiv Hlthcare
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | EPZICOM | abacavir sulfate; lamivudine | TABLET;ORAL | 021652-001 | Aug 2, 2004 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Viiv Hlthcare | CABENUVA KIT | cabotegravir; rilpivirine | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 212888-002 | Jan 21, 2021 | RX | Yes | Yes | 8,410,103 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Viiv Hlthcare | CABENUVA KIT | cabotegravir; rilpivirine | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 212888-001 | Jan 21, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Viiv Hlthcare | CABENUVA KIT | cabotegravir; rilpivirine | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 212888-001 | Jan 21, 2021 | RX | Yes | Yes | 8,410,103 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-003 | Nov 4, 2016 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Viiv Hlthcare | TRIUMEQ PD | abacavir sulfate; dolutegravir sodium; lamivudine | TABLET, FOR SUSPENSION;ORAL | 215413-001 | Mar 30, 2022 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Viiv Hlthcare
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | RESCRIPTOR | delavirdine mesylate | TABLET;ORAL | 020705-001 | Apr 4, 1997 | 5,563,142 | ⤷ Try a Trial |
| Viiv Hlthcare | COMBIVIR | lamivudine; zidovudine | TABLET;ORAL | 020857-001 | Sep 26, 1997 | 6,180,639*PED | ⤷ Try a Trial |
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | 8,101,629 | ⤷ Try a Trial |
| Viiv Hlthcare | ZIAGEN | abacavir sulfate | SOLUTION;ORAL | 020978-001 | Dec 17, 1998 | 6,294,540*PED | ⤷ Try a Trial |
| Viiv Hlthcare | ZIAGEN | abacavir sulfate | SOLUTION;ORAL | 020978-001 | Dec 17, 1998 | 5,089,500*PED | ⤷ Try a Trial |
| Viiv Hlthcare | RETROVIR | zidovudine | INJECTABLE;INJECTION | 019951-001 | Feb 2, 1990 | 4,833,130 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for VIIV HLTHCARE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Oral Solution | 10 mg/mL | ➤ Subscribe | 2011-11-22 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2009-01-28 |
| ➤ Subscribe | Tablets | 700 mg | ➤ Subscribe | 2012-01-18 |
| ➤ Subscribe | Tablets | 600 mg/300 mg | ➤ Subscribe | 2007-09-27 |
| ➤ Subscribe | Tablets | 600 mg/50 mg/300 mg | ➤ Subscribe | 2017-08-14 |
| ➤ Subscribe | Tablets | 150 mg and 300 mg | ➤ Subscribe | 2007-10-16 |
| ➤ Subscribe | Tablets | 150 mg and 300 mg | ➤ Subscribe | 2011-08-08 |
| ➤ Subscribe | Oral Solution | 20 mg/ml | ➤ Subscribe | 2012-12-27 |
| ➤ Subscribe | Tablets | 150 mg/300 mg | ➤ Subscribe | 2007-06-26 |
| ➤ Subscribe | Tablets | 10 mg, 25 mg and 50 mg | ➤ Subscribe | 2017-08-14 |
| ➤ Subscribe | Tablets | 300 mg/150 mg/300 mg | ➤ Subscribe | 2011-03-22 |
International Patents for Viiv Hlthcare Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Lithuania | 3372281 | ⤷ Try a Trial |
| Japan | 2005507380 | ⤷ Try a Trial |
| Taiwan | I315199 | ⤷ Try a Trial |
| Portugal | 1874117 | ⤷ Try a Trial |
| Brazil | PI0508876 | ⤷ Try a Trial |
| Eurasian Patent Organization | 201290583 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Viiv Hlthcare Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0434450 | 33/1999 | Austria | ⤷ Try a Trial | PRODUCT NAME: ''ABACAVIR'', GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES ODER DERIVATS, EINSCHLIESSLICH ABACAVIR-SULFAT.; NAT. REGISTRATION NO/DATE: EU/1/99/112/001 EU/1/99/112/002 19990708; FIRST REGISTRATION: LI 55048 001, 55049 002 19990628 |
| 2465580 | 2190020-4 | Sweden | ⤷ Try a Trial | PRODUCT NAME: CABOTEGRAVIR OR A PHAMACEUTICALLY ACCEPTABLE SALT THEREOF; REG. NO/DATE: EU/1/20/1481 20201221 |
| 3808743 | CR 2022 00035 | Denmark | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION AF RILPIVIRIN ELLER ET FARMACEUTISK ACCEPTABELT ADDITIONSSALT AF RILPIVIRIN, HERUNDER RILPIVIRINHYDROCHLORID, OG EMTRICITABIN; REG. NO/DATE: EU/1/11/737/001-002 20111128 |
| 1874117 | CA 2014 00032 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DOLUTEGRAVIR ELLER ET FARMACEUTISK ACCEPTABELT SALT ELLER SOLVAT DERAF, HERUNDER DOLUTEGRAVIRNATRIUM; REG. NO/DATE: EU/1/13/892/001-002 20140116 |
| 3808743 | 122022000051 | Germany | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION VON RILPIVIRIN ODER EINER THERAPEUTISCH AEQUIVALENTEN FORM DAVON, DIE DURCH DAS GRUNDPATENT GESCHUETZT IST, WIE EIN PHARMAZEUTISCH AKZEPTABLES ADDITIONSSALZ VON RILPIVIRIN, EINSCHLIESSLICH DES SALZSAEURESALZES VON RILPIVIRIN, UND EMTRICITABIN.; REGISTRATION NO/DATE: EU/1/11/737/001-002 20111128 |
| 1419152 | 2012/017 | Ireland | ⤷ Try a Trial | PRODUCT NAME: RILPIVIRINE OR A PHARMACEUTICALLY ACCEPTABLE ADDITION SALT THEREOF, INCLUDING THE HYDROCHLORIC ACID SALT OF RILPIVIRINE; REGISTRATION NO/DATE: EU/1/11/736/001 20111128 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.