Teva Branded Pharm Company Profile
✉ Email this page to a colleague
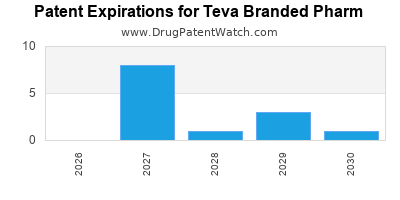
What is the competitive landscape for TEVA BRANDED PHARM, and when can generic versions of TEVA BRANDED PHARM drugs launch?
TEVA BRANDED PHARM has thirty-three approved drugs.
There are fifty-nine US patents protecting TEVA BRANDED PHARM drugs.
There are six hundred and eighty patent family members on TEVA BRANDED PHARM drugs in forty-one countries and thirty-nine supplementary protection certificates in fourteen countries.
Summary for Teva Branded Pharm
| International Patents: | 680 |
| US Patents: | 59 |
| Tradenames: | 33 |
| Ingredients: | 19 |
| NDAs: | 33 |
| Patent Litigation for Teva Branded Pharm: | See patent lawsuits for Teva Branded Pharm |
Drugs and US Patents for Teva Branded Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | LOXITANE | loxapine succinate | TABLET;ORAL | 017525-008 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Branded Pharm | PROAIR HFA | albuterol sulfate | AEROSOL, METERED;INHALATION | 021457-001 | Oct 29, 2004 | AB2 | RX | Yes | Yes | 9,808,587 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Teva Branded Pharm | PROGLYCEM | diazoxide | CAPSULE;ORAL | 017425-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Branded Pharm | AUSTEDO | deutetrabenazine | TABLET;ORAL | 208082-003 | Apr 3, 2017 | RX | Yes | Yes | 11,564,917*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Teva Branded Pharm | AUSTEDO | deutetrabenazine | TABLET;ORAL | 208082-002 | Apr 3, 2017 | RX | Yes | No | 11,357,772*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Teva Branded Pharm | PROAIR RESPICLICK | albuterol sulfate | POWDER, METERED;INHALATION | 205636-001 | Mar 31, 2015 | RX | Yes | Yes | 9,731,087 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Teva Branded Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | PROAIR HFA | albuterol sulfate | AEROSOL, METERED;INHALATION | 021457-001 | Oct 29, 2004 | 5,766,573 | ⤷ Try a Trial |
| Teva Branded Pharm | ZIAC | bisoprolol fumarate; hydrochlorothiazide | TABLET;ORAL | 020186-003 | Mar 26, 1993 | 4,258,062 | ⤷ Try a Trial |
| Teva Branded Pharm | PROAIR HFA | albuterol sulfate | AEROSOL, METERED;INHALATION | 021457-001 | Oct 29, 2004 | 6,352,684 | ⤷ Try a Trial |
| Teva Branded Pharm | QVAR 40 | beclomethasone dipropionate | AEROSOL, METERED;INHALATION | 020911-002 | Sep 15, 2000 | 5,766,573 | ⤷ Try a Trial |
| Teva Branded Pharm | PROAIR HFA | albuterol sulfate | AEROSOL, METERED;INHALATION | 021457-001 | Oct 29, 2004 | 6,446,627 | ⤷ Try a Trial |
| Teva Branded Pharm | ZIAC | bisoprolol fumarate; hydrochlorothiazide | TABLET;ORAL | 020186-001 | Mar 26, 1993 | 4,258,062*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TEVA BRANDED PHARM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 0.1 mg/0.02 mg and 0.01 mg | ➤ Subscribe | 2009-11-16 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg | ➤ Subscribe | 2004-03-29 |
| ➤ Subscribe | Tablets | 1 mg/0.02 mg and 75 mg | ➤ Subscribe | 2006-04-17 |
| ➤ Subscribe | Tablets | 0.15 mg/0.02 mg, 0.15 mg/0.025 mg, 0.15 mg/0.03 mg and 0.01 mg | ➤ Subscribe | 2013-07-10 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg/0.01 mg | ➤ Subscribe | 2008-01-22 |
International Patents for Teva Branded Pharm Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Brazil | 112019018966 | ⤷ Try a Trial |
| South Korea | 102631093 | ⤷ Try a Trial |
| South Korea | 20130103823 | ⤷ Try a Trial |
| Spain | 2907984 | ⤷ Try a Trial |
| Japan | 5497964 | ⤷ Try a Trial |
| Chile | 2019002629 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Teva Branded Pharm Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0503785 | 91330 | Luxembourg | ⤷ Try a Trial | CERTIFICATE TITLE: UNE COMBINAISON D'OLMESARTAN MEDOXOMIL, OPTIONNELLEMENT SOUS LA FORME D'UN SEL PHARMACEUTIQUEMENT ACCEPTABLE ET D'HYDROCHLOROTHIAZIDE (OLMETEC PLUS) |
| 1453521 | C 2015 029 | Romania | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL SI ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: RO 7793/2015/001; DATE OF NATIONAL AUTHORISATION: 20150612; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): SK. 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150129 |
| 1389098 | 300609 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LOXAPINE; REGISTRATION NO/DATE: EU/1/13/823/001-002 20130220 |
| 1453521 | 15C0050 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| 0136011 | 2000C/027 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 0565634 | 06C0030 | France | ⤷ Try a Trial | PRODUCT NAME: EPROSARTAN MESYLATE; HYDROCHLOROTHIAZIDE; NAT. REGISTRATION NO/DATE: NL 32075 20060623; FIRST REGISTRATION: LI - 55783 01 20020607 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.