Taro Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TARO, and when can generic versions of TARO drugs launch?
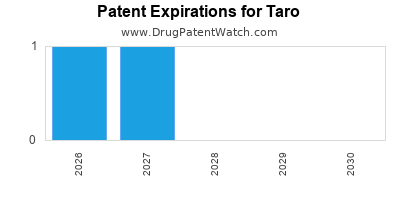
TARO has two hundred and fifty-three approved drugs.
There are eight US patents protecting TARO drugs. There are four tentative approvals on TARO drugs.
There are thirty-one patent family members on TARO drugs in twelve countries and one hundred and seventy-three supplementary protection certificates in fourteen countries.
Summary for Taro
| International Patents: | 31 |
| US Patents: | 8 |
| Tradenames: | 133 |
| Ingredients: | 116 |
| NDAs: | 253 |
| Patent Litigation for Taro: | See patent lawsuits for Taro |
| PTAB Cases with Taro as petitioner: | See PTAB cases with Taro as petitioner |
Drugs and US Patents for Taro
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taro | ETODOLAC | etodolac | TABLET, EXTENDED RELEASE;ORAL | 076174-002 | Mar 13, 2003 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro | CLOBAZAM | clobazam | TABLET;ORAL | 209440-002 | Oct 22, 2018 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Taro | ECONAZOLE NITRATE | econazole nitrate | CREAM;TOPICAL | 076005-001 | Nov 26, 2002 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro | RISPERIDONE | risperidone | SOLUTION;ORAL | 090347-001 | Feb 7, 2011 | AA | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro | LORATADINE | loratadine | SYRUP;ORAL | 076805-001 | Aug 20, 2004 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Taro | FLUOROURACIL | fluorouracil | SOLUTION;TOPICAL | 076526-001 | Nov 5, 2003 | AT | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Taro
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,656,482 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-001 | Jan 17, 2008 | 5,881,926 | ⤷ Try a Trial |
| Taro | TOPICORT | desoximetasone | SPRAY;TOPICAL | 204141-001 | Apr 11, 2013 | 5,990,100 | ⤷ Try a Trial |
| Taro | PLIAGLIS | lidocaine; tetracaine | CREAM;TOPICAL | 021717-001 | Jun 29, 2006 | 5,919,479 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,071,523 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 5,881,926 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TARO drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Topical Lotion | 0.5% | ➤ Subscribe | 2011-03-16 |
| ➤ Subscribe | Topical Spray | 0.25% | ➤ Subscribe | 2013-12-18 |
International Patents for Taro Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Israel | 187666 | ⤷ Try a Trial |
| Mexico | 2012008168 | ⤷ Try a Trial |
| Japan | 5474347 | ⤷ Try a Trial |
| South Africa | 200800926 | ⤷ Try a Trial |
| Brazil | 112012017554 | ⤷ Try a Trial |
| Japan | 2009503097 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Taro Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1458369 | SPC/GB10/005 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ADAPALENE AND BENZOYL PEROXIDE; REGISTERED: DK 40440 20071218; UK PL10590/0057 20091111 |
| 0284288 | SPC/GB98/002 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TAZAROTENE : ETHYL 6-(2-(4,4-DIMETHYLTHIOCHROMAN-6-YL) ETHYNYL) NICOTINOATE; REGISTERED: DE 37393.00.00 19961203; DE 37393.01.00 19961203; UK 00426/0097 19970730; UK 00426/0096 19970730 |
| 0290047 | SPC/GB97/078 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: MANGAFODIPIR AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, IN PARTICULAR MANGAFODIPIR TRISODIUM; REGISTERED: UK EU/1/97/040/001 19970522; UK EU/1/97/040/002 19970522 |
| 1781277 | PA2024501 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: IBUPROFENO IR PARACETAMOLIO DERINYS; REGISTRATION NO/DATE: LT/1/23/5212/001-002 20230726 |
| 1620113 | 56/2015 | Austria | ⤷ Try a Trial | PRODUCT NAME: IVERMECTIN; NAT. REGISTRATION NO/DATE: 136170 20150602; FIRST REGISTRATION: MT MA 117/01101 20150402 |
| 0266730 | SPC/GB97/005 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: DOLASETRON, OR A PHARMACEUTICALLY-ACCEPTABLE ACID ADDITION OR QUATERNARY AMMONIUM SALT THEREOF; REGISTERED: UK 04425/0150 19960926 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.