Sanofi Aventis Us Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANOFI AVENTIS US, and what generic alternatives to SANOFI AVENTIS US drugs are available?
SANOFI AVENTIS US has one hundred and twenty-two approved drugs.
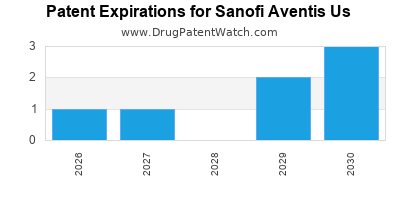
There are ten US patents protecting SANOFI AVENTIS US drugs.
There are two hundred and seventeen patent family members on SANOFI AVENTIS US drugs in fifty-two countries and one hundred and fifteen supplementary protection certificates in nineteen countries.
Summary for Sanofi Aventis Us
| International Patents: | 217 |
| US Patents: | 10 |
| Tradenames: | 97 |
| Ingredients: | 85 |
| NDAs: | 122 |
Drugs and US Patents for Sanofi Aventis Us
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | BRICANYL | terbutaline sulfate | TABLET;ORAL | 017618-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | NEGGRAM | nalidixic acid | TABLET;ORAL | 014214-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | ARAVA | leflunomide | TABLET;ORAL | 020905-003 | Sep 10, 1998 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | DECAPRYN | doxylamine succinate | TABLET;ORAL | 006412-015 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | DEMI-REGROTON | chlorthalidone; reserpine | TABLET;ORAL | 015103-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | AVALIDE | hydrochlorothiazide; irbesartan | TABLET;ORAL | 020758-004 | Mar 15, 2005 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | TALWIN COMPOUND | aspirin; pentazocine hydrochloride | TABLET;ORAL | 016891-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Sanofi Aventis Us
Paragraph IV (Patent) Challenges for SANOFI AVENTIS US drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 5 mg/mL, 10 mL and 20 mL vials | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 200 mg/40 mL | ➤ Subscribe | 2007-07-16 |
| ➤ Subscribe | Injection | 100 mg/mL, 3 mL vials | ➤ Subscribe | 2006-12-07 |
| ➤ Subscribe | Extended-release Tablets | 12.5 mg | ➤ Subscribe | 2006-01-19 |
| ➤ Subscribe | Tablets | 150 mg/12.5 mg and 300 mg/12.5 mg | ➤ Subscribe | 2004-11-10 |
| ➤ Subscribe | Tablets | 75 mg, 150 mg and 300 mg | ➤ Subscribe | 2004-05-25 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2009-03-04 |
| ➤ Subscribe | Injection | 40 mg/mL, 0.5 mL and 2 mL vials | ➤ Subscribe | 2009-06-30 |
| ➤ Subscribe | For Injection | 50 mg/vial and 100 mg/vial | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 5 mg/mL, 40 mL vial | ➤ Subscribe | 2011-03-23 |
| ➤ Subscribe | Extended-release Tablets | 6.25 mg | ➤ Subscribe | 2006-02-24 |
| ➤ Subscribe | Tablets | 7 mg and 14 mg | ➤ Subscribe | 2016-09-12 |
| ➤ Subscribe | Tablets | 300 mg/25 mg | ➤ Subscribe | 2006-06-06 |
| ➤ Subscribe | Tablets | 400 mg | ➤ Subscribe | 2013-07-01 |
International Patents for Sanofi Aventis Us Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Serbia | 54614 | ⤷ Try a Trial |
| Poland | 2477611 | ⤷ Try a Trial |
| Germany | 60226855 | ⤷ Try a Trial |
| China | 100429207 | ⤷ Try a Trial |
| Costa Rica | 8292 | ⤷ Try a Trial |
| Slovenia | 1381356 | ⤷ Try a Trial |
| Uruguay | 32889 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Sanofi Aventis Us Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3300601 | 2022027 | Norway | ⤷ Try a Trial | PRODUCT NAME: COMBINATION OF DAUNORUBICIN AND CYTARABINE; REG. NO/DATE: EU/1/18/1308 20180831 |
| 1381356 | 92366 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: TERIFLUNOMIDE,SON STEREOISOMERE ET LEURS SELS PHARMACEUTIQUEMENT ACCEPTABLES |
| 2236132 | 92636 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: ZOLPIDEM ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES |
| 0443983 | C00443983/03 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: VALSARTAN + AMLODIPINE + HYDROCHLOROTHIAZIDE; REGISTRATION NUMBER/DATE: SWISSMEDIC 59407 16.09.2009 |
| 2768484 | 384 1-2020 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: KOMBINACIA DAUNORUBICINU A CYTARABINU; REGISTRATION NO/DATE: EU/1/18/1308 20180827 |
| 1667986 | 92172 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| 1381356 | 2014C/008 | Belgium | ⤷ Try a Trial | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), CESSION |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.