Sandoz Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANDOZ, and when can generic versions of SANDOZ drugs launch?
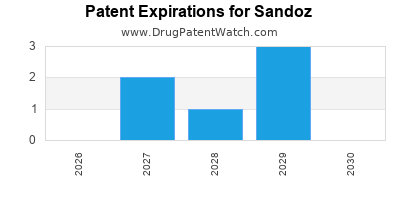
SANDOZ has four hundred and eighty-nine approved drugs.
There are nine US patents protecting SANDOZ drugs. There are thirty-three tentative approvals on SANDOZ drugs.
There are one hundred and three patent family members on SANDOZ drugs in thirty-four countries and five hundred and three supplementary protection certificates in seventeen countries.
Summary for Sandoz
| International Patents: | 103 |
| US Patents: | 9 |
| Tradenames: | 322 |
| Ingredients: | 300 |
| NDAs: | 489 |
| Patent Litigation for Sandoz: | See patent lawsuits for Sandoz |
| PTAB Cases with Sandoz as petitioner: | See PTAB cases with Sandoz as petitioner |
Drugs and US Patents for Sandoz
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sandoz | PEMETREXED DISODIUM | pemetrexed disodium | SOLUTION;INTRAVENOUS | 214657-002 | May 26, 2022 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | NADOLOL | nadolol | TABLET;ORAL | 074501-002 | Nov 9, 1995 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz Inc | ZIPRASIDONE HYDROCHLORIDE | ziprasidone hydrochloride | CAPSULE;ORAL | 077562-003 | Jun 1, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | HEPARIN SODIUM | heparin sodium | INJECTABLE;INJECTION | 091682-002 | Jun 8, 2011 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | PRAMIPEXOLE DIHYDROCHLORIDE | pramipexole dihydrochloride | TABLET, EXTENDED RELEASE;ORAL | 202353-003 | Dec 4, 2014 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | CIMETIDINE | cimetidine | TABLET;ORAL | 075122-001 | Jun 19, 1998 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | CARBOPLATIN | carboplatin | INJECTABLE;INJECTION | 076959-002 | Mar 18, 2005 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Sandoz
Paragraph IV (Patent) Challenges for SANDOZ drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Ophthalmic Emulsion | 0.05% | ➤ Subscribe | 2014-05-01 |
| ➤ Subscribe | Oral Solution | 2 mg/mL | ➤ Subscribe | 2004-11-05 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Oral Solution | 4 mg/5 mL | ➤ Subscribe | 2004-12-20 |
| ➤ Subscribe | Extended-release Capsules | 5mg, 10mg and 20 mg | ➤ Subscribe | 2007-03-30 |
| ➤ Subscribe | Injection | 0.05 mg/mL, 100 mL vial | ➤ Subscribe | 2008-08-29 |
| ➤ Subscribe | Extended-release Capsule | 40 mg | ➤ Subscribe | 2010-12-20 |
| ➤ Subscribe | Otic Suspension | 0.3%/0.1% | ➤ Subscribe | 2012-07-31 |
| ➤ Subscribe | Extended-release capsules | 35 mg | ➤ Subscribe | 2011-09-29 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2004-07-27 |
| ➤ Subscribe | Extended-release Capsules | 20 mg, 30 mg and 40 mg | ➤ Subscribe | 2006-08-21 |
| ➤ Subscribe | Capsules | 5 mg/40 mg and 10 mg/40 mg | ➤ Subscribe | 2006-11-17 |
| ➤ Subscribe | Capsules | 1.5 mg, 3 mg, 4.5 mg and 6 mg | ➤ Subscribe | 2004-04-21 |
| ➤ Subscribe | Transdermal System Extended-release | 13.3 mg/24 hr | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
| ➤ Subscribe | Extended-release Capsules | 15 mg | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | For Injection | 250 mg/vial | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Extended-release Capsule | 30 mg | ➤ Subscribe | 2010-12-15 |
| ➤ Subscribe | Ophthalmic Solution | 0.00% | ➤ Subscribe | 2009-02-19 |
| ➤ Subscribe | Extended-release capsules | 25 mg | ➤ Subscribe | 2011-09-30 |
| ➤ Subscribe | Extended-release Tablets | 80 mg | ➤ Subscribe | 2007-03-15 |
| ➤ Subscribe | Extended-release Capsules | 10 mg | ➤ Subscribe | 2007-05-21 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2004-05-27 |
| ➤ Subscribe | Capsules | 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg and 10 mg/20 mg | ➤ Subscribe | 2004-06-09 |
International Patents for Sandoz Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| South Africa | 200901702 | ⤷ Try a Trial |
| European Patent Office | 1689400 | ⤷ Try a Trial |
| European Patent Office | 2066300 | ⤷ Try a Trial |
| Russian Federation | 2004111984 | ⤷ Try a Trial |
| Cyprus | 1112138 | ⤷ Try a Trial |
| Russian Federation | 2295346 | ⤷ Try a Trial |
| Japan | 5394927 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Sandoz Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0720599 | 122014000088 | Germany | ⤷ Try a Trial | PRODUCT NAME: EZETIMIB ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON IN KOMBINATION MIT ATORVASTATIN ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON, INSBESONDERE ATORVASTATIN ALS ATORVASTATINCALCIUMTRIHYDRAT; NAT. REGISTRATION NO/DATE: 90223.00.00 20141113; FIRST REGISTRATION: FRANKREICH CIS: 6 928 917 6 20140912 |
| 0720599 | SPC/GB05/010 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF IN COMBINATION WITH SIMVASTATIN; REGISTERED: DE 58874.00.00 20040402; DE 58874.01.00 20040402; DE 58874.02.00 20040402; DE 58874.03.00 20040402; DE 58878.00.00 20040402; DE 58878.01.00 20040402; DE 58878.02.00 20040402; DE 58878.03.00 20040402; DE 58866.00.00 20040402; DE 58866.01.00 20040402; DE 58866.02.00 20040402; DE 58866.03.00 20040402; DE 58870.00.00 20040402; DE 58870.01.00 20040402; DE 58870.02.00 20040402; DE 58870.03.00 20040402; UK PL 19945/0003 20041118; UK PL 19945/0004 20041118; UK PL 19945/0005 20041118; UK PL 19945/0006 20041118; UK PL 19945/0007 20041118; UK PL 19945/0008 20041118; UK PL 19945/0009 200411 |
| 1506211 | 132014902277722 | Italy | ⤷ Try a Trial | PRODUCT NAME: UNA COMBINAZIONE DI DAPAGLIFLOZIN O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE E METFORMINA O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE COME PROTETTI DAL BREVETTO DI BASE EP1506211(XIGDUO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/13/900, 20140116 |
| 1280520 | CA 2015 00017 | Denmark | ⤷ Try a Trial | PRODUCT NAME: TOBRAMYCIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/10/652/001-003 20110720 |
| 2498758 | CA 2020 00017 | Denmark | ⤷ Try a Trial | PRODUCT NAME: METFORMIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; SAXAGLIPTIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; DAPAGLIFLOZIN ELLER ET FARMACEUTISK ACCEPTABELT SOLVAT DERAF; REG. NO/DATE: EU/1/19/1401 20191113 |
| 1514548 | C300671 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TRAVOPROST; REGISTRATION NO/DATE: EG EU/1/01/199/001-002 20011128 |
| 0166287 | 96C0032 | Belgium | ⤷ Try a Trial | PRODUCT NAME: PANTOPRAZOL. NATR. SESQUIHYDRAS PANTOPRAZOLE; NAT. REGISTRATION NO/DATE: 127 IS 98 F 3 19960222; FIRST REGISTRATION: SE 12131 19940506 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.