Mallinckrodt Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for MALLINCKRODT INC, and what generic alternatives to MALLINCKRODT INC drugs are available?
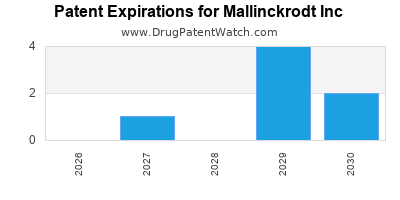
MALLINCKRODT INC has four approved drugs.
There are twelve US patents protecting MALLINCKRODT INC drugs.
There are thirty-one patent family members on MALLINCKRODT INC drugs in thirteen countries and sixty-seven supplementary protection certificates in eight countries.
Summary for Mallinckrodt Inc
| International Patents: | 31 |
| US Patents: | 12 |
| Tradenames: | 4 |
| Ingredients: | 4 |
| NDAs: | 4 |
| Drug Master File Entries: | 67 |
Drugs and US Patents for Mallinckrodt Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 8,394,408 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 8,658,631 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 8,372,432 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 9,050,335 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mallinckrodt Inc | METHADONE HYDROCHLORIDE | methadone hydrochloride | POWDER;FOR RX COMPOUNDING | 006383-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Mallinckrodt Inc | METHADONE HYDROCHLORIDE | methadone hydrochloride | POWDER;FOR RX COMPOUNDING | 006383-004 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 9,468,636 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Mallinckrodt Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | 6,488,962 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for MALLINCKRODT INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 7.5 mg/325 mg | ➤ Subscribe | 2014-04-03 |
Premature patent expirations for MALLINCKRODT INC
Expiration due to failure to pay maintenance fee
| Patent Number | Expiration Date |
|---|---|
| ⤷ Try a Trial | ⤷ Try a Trial |
International Patents for Mallinckrodt Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2011087765 | ⤷ Try a Trial |
| Hong Kong | 1152469 | ⤷ Try a Trial |
| Brazil | 112013015622 | ⤷ Try a Trial |
| Japan | 5607550 | ⤷ Try a Trial |
| China | 102105136 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2009114648 | ⤷ Try a Trial |
| European Patent Office | 1439826 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Mallinckrodt Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1499331 | SPC/GB13/034 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: IZINOVA CONCENTRATE FOR ORAL SOLUTION. THE ACTIVE SUBSTANCE IS A MIXTURE OF 3 SALTS:SODIUM SULPHATE ANHYDROUS, MAGNESIUM SULPHATE HEPTAHYDRATE AND POTASSIUM SULPHATE.; REGISTERED: BE BE434323 20130220; UK PL34926/0016 20130313 |
| 2465580 | SPC/GB21/030 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: CABOTEGRAVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF, INCLUDING CABOTEGRAVIR SODIUM.; REGISTERED: UK EU/1/20/1481 (NI) 20201221; UK PLGB 35728/0055-57 20201221 |
| 2203431 | CR 2015 00014 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REG. NO/DATE: EU/1/14/983 20150119 |
| 2673237 | LUC00111 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SODIUM ZIRCONIUM CYCLOSILICATE; AUTHORISATION NUMBER AND DATE: EU/1/17/1173 20180326 |
| 2203431 | CA 2015 00014 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REG. NO/DATE: EU/1/14/983 20150115 |
| 2666774 | CA 2020 00037 | Denmark | ⤷ Try a Trial | PRODUCT NAME: RELEBACTAM, OPTIONALLY IN THE FORM OF THE MONOHYDRATE, IMIPENEM AND CILASTATIN, OPTIONALLY IN THE FORM OF THE SODIUM SALT; REG. NO/DATE: EU/1/19/1420 20200217 |
| 2932970 | 1890039-9 | Sweden | ⤷ Try a Trial | PRODUCT NAME: DOLUTEGRAVIR SODIUM + RILPIVIRINE HYDROCHLORIDE; REG. NO/DATE: EU/1/18/1282 20180518 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.