Galderma Labs Lp Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GALDERMA LABS LP, and when can generic versions of GALDERMA LABS LP drugs launch?
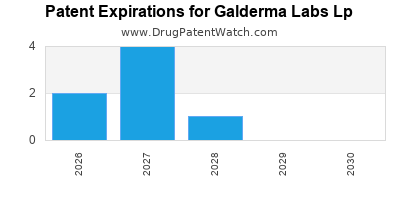
GALDERMA LABS LP has twenty-six approved drugs.
There are fifty-eight US patents protecting GALDERMA LABS LP drugs.
There are four hundred and five patent family members on GALDERMA LABS LP drugs in thirty-seven countries and one hundred and seventy-eight supplementary protection certificates in eighteen countries.
Summary for Galderma Labs Lp
| International Patents: | 405 |
| US Patents: | 58 |
| Tradenames: | 18 |
| Ingredients: | 16 |
| NDAs: | 26 |
Drugs and US Patents for Galderma Labs Lp
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | TWYNEO | benzoyl peroxide; tretinoin | CREAM;TOPICAL | 214902-001 | Jul 26, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Galderma Labs Lp | EPSOLAY | benzoyl peroxide | CREAM;TOPICAL | 214510-001 | Apr 22, 2022 | RX | Yes | Yes | 11,541,026 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | EPSOLAY | benzoyl peroxide | CREAM;TOPICAL | 214510-001 | Apr 22, 2022 | RX | Yes | Yes | 11,877,997 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | DESOWEN | desonide | CREAM;TOPICAL | 019048-001 | Dec 14, 1984 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | METROGEL | metronidazole | GEL;TOPICAL | 021789-001 | Jun 30, 2005 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | SOOLANTRA | ivermectin | CREAM;TOPICAL | 206255-001 | Dec 19, 2014 | AB | RX | Yes | Yes | 11,033,565 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Galderma Labs Lp | DIFFERIN | adapalene | LOTION;TOPICAL | 022502-001 | Mar 17, 2010 | RX | Yes | Yes | 8,435,502 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Galderma Labs Lp
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | METVIXIA | methyl aminolevulinate hydrochloride | CREAM;TOPICAL | 021415-001 | Jul 27, 2004 | 6,034,267 | ⤷ Try a Trial |
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | 8,105,618 | ⤷ Try a Trial |
| Galderma Labs Lp | DIFFERIN | adapalene | SOLUTION;TOPICAL | 020338-001 | May 31, 1996 | RE34440 | ⤷ Try a Trial |
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | 8,603,506 | ⤷ Try a Trial |
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | 8,394,406 | ⤷ Try a Trial |
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | 5,919,775 | ⤷ Try a Trial |
| Galderma Labs Lp | SOOLANTRA | ivermectin | CREAM;TOPICAL | 206255-001 | Dec 19, 2014 | 7,550,440 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GALDERMA LABS LP drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Capsules | 40 mg | ➤ Subscribe | 2008-12-11 |
| ➤ Subscribe | Cream | 1% | ➤ Subscribe | 2016-12-30 |
| ➤ Subscribe | Topical Gel | 1% | ➤ Subscribe | 2008-10-21 |
| ➤ Subscribe | Lotion | 0.05% | ➤ Subscribe | 2006-03-27 |
| ➤ Subscribe | Topical Shampoo | 0.05% | ➤ Subscribe | 2008-01-09 |
| ➤ Subscribe | Topical Gel | 0.30% | ➤ Subscribe | 2009-09-15 |
| ➤ Subscribe | Gel | 0.1%/2.5% | ➤ Subscribe | 2011-12-30 |
| ➤ Subscribe | Topical Gel | 0.33% | ➤ Subscribe | 2014-12-15 |
| ➤ Subscribe | Spray | 0.05% | ➤ Subscribe | 2008-09-29 |
International Patents for Galderma Labs Lp Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Denmark | 1761266 | ⤷ Try a Trial |
| Japan | 2005513146 | ⤷ Try a Trial |
| South Africa | 201402758 | ⤷ Try a Trial |
| Mexico | PA05011208 | ⤷ Try a Trial |
| China | 1607946 | ⤷ Try a Trial |
| Germany | 122008000041 | ⤷ Try a Trial |
| Russian Federation | 2014152998 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Galderma Labs Lp Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1667986 | 92172 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| 1131065 | PA2014023 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DIMETHYLIS FUMARAS; REGISTRATION NO/DATE: EU/1/13/837/001-002 20140130 |
| 1532149 | CA 2013 00001 | Denmark | ⤷ Try a Trial | PRODUCT NAME: 8-(3-AMINOPIPERIDIN-1-YL)-7-BUT-2-INYL-3-METHYL-1-(4-METHYLCHINAZOLIN-2-YLMETHYL)-3,7-DIHYDROPURIN-2,6-DION ENANTIOMERER OG SALTE DERAF - SAERLIGT LINAGLIPTIN - I KOMBINATION MED METFORMINHYDROCHLORID; REG. NO/DATE: EU/1/12/780/001-028 20120720 |
| 1948158 | 2016C/026 | Belgium | ⤷ Try a Trial | PRODUCT NAME: SACUBITRIL/VALSARTAN, ALS SACUBITRIL VALSARTAN NATRIUMZOUT COMPLEX, I.E. TRINATRIUM (3-((1S,3R)-1-BIFENYL-4-YLMETHYL-3-ETHOXYCARBONIL-1-BUTYLCARBAMOYL)PROPIONAAT-(S)-3'-METHYL-2'-(PENTANOYL(2-(TETRAZOL-5-YLATE)BIFENYL-4'-YLMETHYL)AMINO)BUTYRAAT)HEMIPENTAHYDRAAT; AUTHORISATION NUMBER AND DATE: EU/1/15/1058 20151123 |
| 1458369 | SZ 31/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: KOMBINATIONSPRAEPARAT ENTHALTEND ADAPALEN UND BENZOLYPEROXID |
| 0733366 | SPC/GB98/031 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: 2-BUTYL-4-CHLORO-1-((2'-(1H-TETRAZOL-5-YL) BIPHENYL-4-YL)-METHYL)-5-(HYDROXYMETHYL)IMIDAZOLE (LOSARTAN),OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, IN PARTICULAR A POTASSIUM SALT( LOSARTAN POTASSIUM) AND HYDROCHLOROTHIAZIDE,; REGISTERED: FR 338520.7 19950215; FR 558453.7 19950215; UK 00025/0338 19960412 |
| 1532149 | 2011C/038 | Belgium | ⤷ Try a Trial | PRODUCT NAME: 8-(3-AMINOPIPERIDIN-1-YL)-7-BUT-2-INYL-3-METHYL-1-(4-METHYLCHINAZOLIN-2--YLMETHYL)-3,7-DIHYDROPURIN-2,6-DIONE SES ENANTIOMERES OU L'UN DES SES SELS, EN PARTICULIER LA LINAGLIPTINE; AUTHORISATION NUMBER AND DATE: EU/1/11/707/001 20110830 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.