Nitroglycerin - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for nitroglycerin and what is the scope of patent protection?
Nitroglycerin
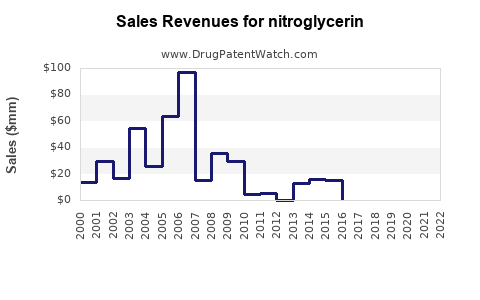
is the generic ingredient in sixteen branded drugs marketed by Evus, Pohl Boskamp, Bausch, Valeant Pharms, Valeant Pharms North, Uspharma, Lannett Co Inc, Mylan Technologies, Zydus Pharms, Novartis, Sanofi Aventis Us, Abraxis Pharm, Am Regent, Hospira, Intl Medication, Luitpold, Smith And Nephew, Baxter Hlthcare, Rorer, Parke Davis, Cosette, Abbvie, Fougera Pharms Inc, Padagis Israel, Actavis Labs Fl Inc, Aurobindo Pharma, Dr Reddys, Glenmark Pharms Sa, Mankind Pharma, Natco, Rubicon, Sigmapharm Labs Llc, and Viatris, and is included in fifty-one NDAs. There are two patents protecting this compound and four Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Nitroglycerin has twenty patent family members in nine countries.
There are thirty-six drug master file entries for nitroglycerin. Thirty-three suppliers are listed for this compound. There are three tentative approvals for this compound.
Summary for nitroglycerin
| International Patents: | 20 |
| US Patents: | 2 |
| Tradenames: | 16 |
| Applicants: | 33 |
| NDAs: | 51 |
| Drug Master File Entries: | 36 |
| Finished Product Suppliers / Packagers: | 33 |
| Raw Ingredient (Bulk) Api Vendors: | 36 |
| Clinical Trials: | 184 |
| Patent Applications: | 6,357 |
| Formulation / Manufacturing: | see details |
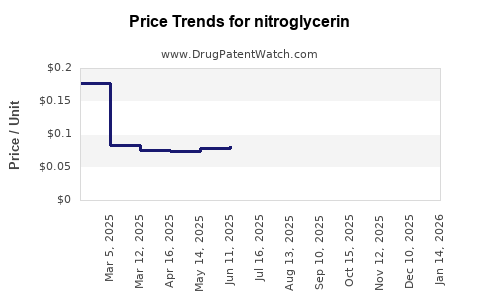
| Drug Prices: | Drug price trends for nitroglycerin |
| What excipients (inactive ingredients) are in nitroglycerin? | nitroglycerin excipients list |
| DailyMed Link: | nitroglycerin at DailyMed |
Recent Clinical Trials for nitroglycerin
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Alabama at Birmingham | Early Phase 1 |
| Tanta University | N/A |
| Pak Emirates Military Hospital | N/A |
Generic filers with tentative approvals for NITROGLYCERIN
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 0.2MG/HR | Film, Extended Release; Transdermal |
| ⤷ Try a Trial | ⤷ Try a Trial | 0.4MG/HR | Film, Extended Release; Transdermal |
| ⤷ Try a Trial | ⤷ Try a Trial | 0.6MG/HR | Film, Extended Release; Transdermal |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for nitroglycerin
| Drug Class | Nitrate Vasodilator |
| Physiological Effect | Vasodilation |
Medical Subject Heading (MeSH) Categories for nitroglycerin
Paragraph IV (Patent) Challenges for NITROGLYCERIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NITROLINGUAL PUMPSPRAY | Sublingual Spray | nitroglycerin | 400 mcg/spray, 4.9 g and 12 g bottles | 018705 | 1 | 2012-04-17 |
| NITROSTAT | Sublingual Tablets | nitroglycerin | 0.3 mg, 0.4 mg, and 0.6 mg | 021134 | 1 | 2005-10-19 |
US Patents and Regulatory Information for nitroglycerin
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baxter Hlthcare | NITROGLYCERIN IN DEXTROSE 5% | nitroglycerin | INJECTABLE;INJECTION | 019970-003 | Dec 29, 1989 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mylan Technologies | NITROGLYCERIN | nitroglycerin | FILM, EXTENDED RELEASE;TRANSDERMAL | 074992-001 | Nov 12, 1999 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mylan Technologies | NITROGLYCERIN | nitroglycerin | FILM, EXTENDED RELEASE;TRANSDERMAL | 074992-003 | Nov 12, 1999 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sigmapharm Labs Llc | NITROGLYCERIN | nitroglycerin | TABLET;SUBLINGUAL | 207745-003 | May 7, 2018 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lannett Co Inc | NITROGLYCERIN | nitroglycerin | FILM, EXTENDED RELEASE;TRANSDERMAL | 075115-001 | Aug 10, 2004 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for nitroglycerin
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | RECTIV | nitroglycerin | OINTMENT;INTRA-ANAL | 021359-001 | Jun 21, 2011 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | TRANSDERM-NITRO | nitroglycerin | FILM, EXTENDED RELEASE;TRANSDERMAL | 020144-005 | Feb 27, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | TRANSDERM-NITRO | nitroglycerin | FILM, EXTENDED RELEASE;TRANSDERMAL | 020144-001 | Feb 27, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Uspharma | NITRO-DUR | nitroglycerin | FILM, EXTENDED RELEASE;TRANSDERMAL | 020145-006 | Apr 4, 1995 | ⤷ Try a Trial | ⤷ Try a Trial |
| Uspharma | NITRO-DUR | nitroglycerin | FILM, EXTENDED RELEASE;TRANSDERMAL | 020145-002 | Apr 4, 1995 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for nitroglycerin
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 3354259 | GRANULES STABILISÉES CONTENANT DU TRINITRATE DE GLYCÉRYL (STABILIZED GRANULES CONTAINING GLYCERYL TRINITRATE) | ⤷ Try a Trial |
| European Patent Office | 2678000 | GRANULES STABILISÉS CONTENANT DU TRINITRATE DE GLYCÉRYLE (STABILIZED GRANULES CONTAINING GLYCERYL TRINITRATE) | ⤷ Try a Trial |
| Australia | 2012219926 | Stabilized granules containing glyceryl trinitrate | ⤷ Try a Trial |
| Canada | 2718345 | PREPARATION PHARMACEUTIQUE A GRANDE STABILITE, CONTENANT LE PRINCIPE ACTIF TRINITRATE DE GLYCEROL (LONG-TERM STABLE PHARMACEUTICAL PREPARATION HAVING THE ACTIVE INGREDIENT GLYCEROL TRINITRATE) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2012113564 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.