Taro Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TARO, and when can generic versions of TARO drugs launch?
TARO has two hundred and fifty-three approved drugs.
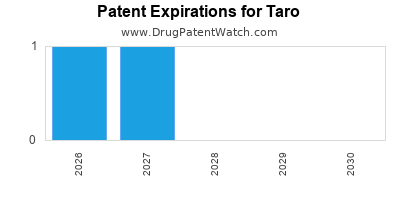
There are eight US patents protecting TARO drugs. There are four tentative approvals on TARO drugs.
There are thirty-one patent family members on TARO drugs in twelve countries and one hundred and seventy-three supplementary protection certificates in fourteen countries.
Summary for Taro
| International Patents: | 31 |
| US Patents: | 8 |
| Tradenames: | 133 |
| Ingredients: | 116 |
| NDAs: | 253 |
| Patent Litigation for Taro: | See patent lawsuits for Taro |
| PTAB Cases with Taro as petitioner: | See PTAB cases with Taro as petitioner |
Drugs and US Patents for Taro
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taro | LORATADINE | loratadine | SUSPENSION;ORAL | 021734-001 | Oct 4, 2005 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Taro | CARVEDILOL | carvedilol | TABLET;ORAL | 077780-002 | Sep 5, 2007 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro | MEPROBAMATE | meprobamate | TABLET;ORAL | 200998-001 | May 23, 2011 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Taro | FELBAMATE | felbamate | TABLET;ORAL | 207093-001 | Apr 20, 2017 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro | FLUOCINONIDE | fluocinonide | CREAM;TOPICAL | 019117-001 | Jun 26, 1984 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Taro
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,656,482 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-001 | Jan 17, 2008 | 5,881,926 | ⤷ Try a Trial |
| Taro | TOPICORT | desoximetasone | SPRAY;TOPICAL | 204141-001 | Apr 11, 2013 | 5,990,100 | ⤷ Try a Trial |
| Taro | PLIAGLIS | lidocaine; tetracaine | CREAM;TOPICAL | 021717-001 | Jun 29, 2006 | 5,919,479 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,071,523 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TARO drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Topical Spray | 0.25% | ➤ Subscribe | 2013-12-18 |
| ➤ Subscribe | Topical Lotion | 0.5% | ➤ Subscribe | 2011-03-16 |
International Patents for Taro Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 1898707 | ⤷ Try a Trial |
| Canada | 2609579 | ⤷ Try a Trial |
| Mexico | 2012008168 | ⤷ Try a Trial |
| China | 103087095 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2011088333 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Taro Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1304992 | 1390049-3 | Sweden | ⤷ Try a Trial | PRODUCT NAME: KLINDAMYCIN (SOM KLINDAMYCINFOSFAT) OCH TRETINOIN; NAT. REG. NO/DATE: MTNR 46193 20130503; FIRST REG.: IR PA1332/043/001 20130322 |
| 2233112 | 132014902285293 | Italy | ⤷ Try a Trial | PRODUCT NAME: FLUOCINOLONE ACETONIDE(ILUVIEN); AUTHORISATION NUMBER(S) AND DATE(S): 042616019, 20140530;PL27813/0001, 20120504 |
| 0347066 | 42/2002 | Austria | ⤷ Try a Trial | PRODUCT NAME: ESCITALOPRAM UND DESSEN NICHT-TOXISCHE SAEUREADDITIONSSALZE; NAT. REGISTRATION NO/DATE: 1-24549, 1-24550, 1-24551, 1-24552 20020618; FIRST REGISTRATION: SE 17084, 17085, 17086,17087 20011207 |
| 2340828 | LUC00195 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SACUBITRIL ET VALSARTAN, SOUS FORME DE COMPLEXE DE SEL DE SODIUM SACUBITRIL VALSARTAN, C'EST-A-DIRE (((S)-N-VALERYL-N-((2'-(1H-TETRAZOLE-5-YL)-BIPHENYL-4-YL)-METHYL)-VALINE) ((2R,4S)-5-BIPHENYL-4-YL-4-(3-CARBOXY-PROPIONYLAMINO)-2-METHYL-ESTER ETHYLIQUE D'ACIDE PENTANOIQUE))NA3 X H2O, DANS LEQUEL X EST 0 A 3; AUTHORISATION NUMBER AND DATE: EU/1/15/1058 20151123 |
| 1620113 | 122015000079 | Germany | ⤷ Try a Trial | PRODUCT NAME: IVERMECTIN ZU SEINER VERWENDUNG FUER DIE BEHANDLUNG VON ROSAZEA; NAT. REGISTRATION NO/DATE: 92429.00.00 20150429; FIRST REGISTRATION: MALTA MA 117/01101 20150402 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.