Takeda Pharms Usa Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TAKEDA PHARMS USA, and when can generic versions of TAKEDA PHARMS USA drugs launch?
TAKEDA PHARMS USA has forty approved drugs.
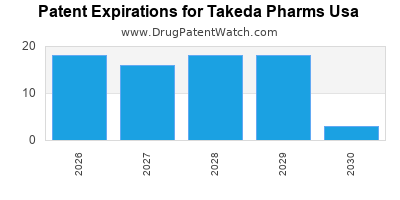
There are one hundred and six US patents protecting TAKEDA PHARMS USA drugs.
There are one thousand four hundred and twenty-seven patent family members on TAKEDA PHARMS USA drugs in fifty-nine countries and one hundred and eighty-eight supplementary protection certificates in nineteen countries.
Summary for Takeda Pharms Usa
| International Patents: | 1427 |
| US Patents: | 106 |
| Tradenames: | 43 |
| Ingredients: | 33 |
| NDAs: | 40 |
Drugs and US Patents for Takeda Pharms Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | OMONTYS PRESERVATIVE FREE | peginesatide acetate | SOLUTION;INTRAVENOUS, SUBCUTANEOUS | 202799-001 | Mar 27, 2012 | DISCN | No | No | 7,919,461 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Takeda Pharms Usa | VYVANSE | lisdexamfetamine dimesylate | TABLET, CHEWABLE;ORAL | 208510-002 | Jan 28, 2017 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Takeda Pharms Usa | DEXILANT | dexlansoprazole | CAPSULE, DELAYED RELEASE;ORAL | 022287-001 | Jan 30, 2009 | AB | RX | Yes | No | 8,173,158*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Takeda Pharms Usa | OMONTYS PRESERVATIVE FREE | peginesatide acetate | SOLUTION;INTRAVENOUS, SUBCUTANEOUS | 202799-003 | Mar 27, 2012 | DISCN | No | No | 7,919,118 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-004 | Sep 30, 2013 | RX | Yes | Yes | 7,144,884*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-003 | Sep 30, 2013 | DISCN | Yes | No | 11,458,134*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Takeda Pharms Usa | FRUZAQLA | fruquintinib | CAPSULE;ORAL | 217564-002 | Nov 8, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Takeda Pharms Usa
Paragraph IV (Patent) Challenges for TAKEDA PHARMS USA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Orally Disinte | 15 mg and 30 mg | ➤ Subscribe | 2006-12-27 |
| ➤ Subscribe | Tablets | 12.5 mg/500 mg and 12.5 mg/1000 mg | ➤ Subscribe | 2017-01-25 |
| ➤ Subscribe | Delayed-release Capsule | 30 mg | ➤ Subscribe | 2010-11-30 |
| ➤ Subscribe | Tablets | 8 mg | ➤ Subscribe | 2009-07-22 |
| ➤ Subscribe | Extended-release Capsules | 37.5 mg and50 mg | ➤ Subscribe | 2017-08-03 |
| ➤ Subscribe | Tablets | 40 mg and 80 mg | ➤ Subscribe | 2013-02-13 |
| ➤ Subscribe | Capsules | 20 mg, 30 mg, 40 mg, 50 mg, 60 mg and 70 mg | ➤ Subscribe | 2011-02-23 |
| ➤ Subscribe | Injection | 10 mg/mL | ➤ Subscribe | 2015-08-25 |
| ➤ Subscribe | Oral Powder | 750 mg and 1000 mg | ➤ Subscribe | 2015-11-25 |
| ➤ Subscribe | Extended-release Tablets | 15 mg/1000 mg and 30 mg/1000 mg | ➤ Subscribe | 2011-09-23 |
| ➤ Subscribe | Tablets | 15 mg/500 mg and 15 mg/850 mg | ➤ Subscribe | 2008-03-06 |
| ➤ Subscribe | Delayed-release Pellets/Capsul | 15 mg and 30 mg | ➤ Subscribe | 2005-12-05 |
| ➤ Subscribe | Tablets | 30 mg/2 mg and 30 mg/4 mg | ➤ Subscribe | 2009-12-22 |
| ➤ Subscribe | Capsule | 60 mg | ➤ Subscribe | 2010-08-25 |
| ➤ Subscribe | Tablets | 6.25 mg, 12.5 mg and 25 mg | ➤ Subscribe | 2017-01-25 |
| ➤ Subscribe | Extended-release Capsules | 100 mg and 200 mg | ➤ Subscribe | 2006-02-02 |
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 15 mgand 20 mg | ➤ Subscribe | 2017-10-02 |
| ➤ Subscribe | Extended-release Capsules | 12.5 mg and 25 mg | ➤ Subscribe | 2017-08-07 |
| ➤ Subscribe | For Injection | 3.5 mg/vial | ➤ Subscribe | 2008-11-20 |
| ➤ Subscribe | Chewable Tablet | 500 mg, 750 mg and 1000 mg | ➤ Subscribe | 2008-10-27 |
| ➤ Subscribe | Delayed-release Tablets | 1.2 g | ➤ Subscribe | 2009-12-16 |
| ➤ Subscribe | Extended-release Tablets | 1 mg, 2 mg, 3 mg and 4 mg | ➤ Subscribe | 2009-12-29 |
| ➤ Subscribe | Tablets | 0.6 mg | ➤ Subscribe | 2011-12-23 |
International Patents for Takeda Pharms Usa Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Peru | 20110019 | ⤷ Try a Trial |
| Australia | 2007260355 | ⤷ Try a Trial |
| Malaysia | 146290 | ⤷ Try a Trial |
| New Zealand | 585247 | ⤷ Try a Trial |
| United Kingdom | 0911779 | ⤷ Try a Trial |
| Croatia | P20120144 | ⤷ Try a Trial |
| Chile | 2017000979 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Takeda Pharms Usa Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2178888 | LUC00013 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: IXAZOMIB ET SELS ET ESTERS PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI, TEL QUE LE CITRATE D'IXAZOMIB; AUTHORISATION NUMBER AND DATE: EU/1/16/&1094 20161123 |
| 2435024 | 2021C/518 | Belgium | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE FORMOTEROL (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES), GLYCOPYRROLATE (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES) ET BUDESONIDE (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/20/1498 20201210 |
| 0193256 | SPC/GB01/016 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: PIOGLITAZONE OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, ESPECIALLY THE HYDROCHLORIDE SALT; REGISTERED: CH IKS 55 378 01 20000927; CH IKS 55 378 02 20000927; CH IKS 55 378 03 20000927; UK EU/1/00/151/001 20001011; UK EU/1/00/151/002 20001011; UK EU/1/00/151/003 20001011; UK EU/1/00/151/004 20001011; UK EU/1/00/151/005 20001011; UK EU/1/00/151/006 20001011 |
| 1973545 | C 2013 039 | Romania | ⤷ Try a Trial | PRODUCT NAME: PONATINIB SI SARURILE SALE ACCEPTABILE FARMACEUTIC; NATIONAL AUTHORISATION NUMBER: EU/1/13/839/001, EU/1/13/839/002, EU/1/13/839/003, EU/1/13/839/004; DATE OF NATIONAL AUTHORISATION: 20130701; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/839/001, EU/1/13/839/002, EU/1/13/839/003, EU/1/13/839/004; DATE OF FIRST AUTHORISATION IN EEA: 20130701 |
| 1436271 | 14C0033 | France | ⤷ Try a Trial | PRODUCT NAME: VORTIOXETINE OU L'UN DE SES SELS D'ADDITION D'ACIDE PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/13/891 20131218 |
| 1586571 | CR 2014 00011 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ALOGLIPTIN; REG. NO/DATE: EU/1/13/844/001-027 20130923 |
| 2300013 | 122019000046 | Germany | ⤷ Try a Trial | PRODUCT NAME: BRIGATINIB, ODER EIN PHARMAZEUTISCH UNBEDENKLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/18/1264 20181122 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.