Bausch And Lomb Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BAUSCH AND LOMB, and when can generic versions of BAUSCH AND LOMB drugs launch?
BAUSCH AND LOMB has eighty-two approved drugs.
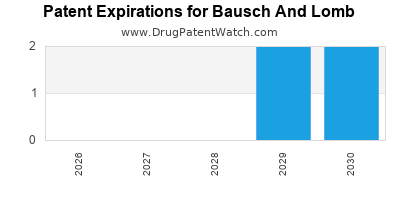
There are forty-nine US patents protecting BAUSCH AND LOMB drugs.
There are four hundred and seventy-one patent family members on BAUSCH AND LOMB drugs in forty-seven countries and eighty supplementary protection certificates in fifteen countries.
Summary for Bausch And Lomb
| International Patents: | 471 |
| US Patents: | 49 |
| Tradenames: | 75 |
| Ingredients: | 58 |
| NDAs: | 82 |
Drugs and US Patents for Bausch And Lomb
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch And Lomb | TIMOLOL MALEATE | timolol maleate | SOLUTION/DROPS;OPHTHALMIC | 074778-001 | Mar 25, 1997 | AT1 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bausch And Lomb Inc | METHAZOLAMIDE | methazolamide | TABLET;ORAL | 207438-002 | Oct 5, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bausch And Lomb Inc | TIMOPTIC | timolol maleate | SOLUTION/DROPS;OPHTHALMIC | 018086-002 | Approved Prior to Jan 1, 1982 | AT1 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bausch And Lomb Inc | MACUGEN | pegaptanib sodium | INJECTABLE;INTRAVITREAL | 021756-001 | Dec 17, 2004 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bausch And Lomb Inc | XIIDRA | lifitegrast | SOLUTION/DROPS;OPHTHALMIC | 208073-001 | Jul 11, 2016 | AB | RX | Yes | Yes | 9,353,088 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bausch And Lomb | OPCON-A | naphazoline hydrochloride; pheniramine maleate | SOLUTION/DROPS;OPHTHALMIC | 020065-001 | Jun 8, 1994 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bausch And Lomb
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch And Lomb Inc | MACUGEN | pegaptanib sodium | INJECTABLE;INTRAVITREAL | 021756-001 | Dec 17, 2004 | 6,426,335 | ⤷ Try a Trial |
| Bausch And Lomb | LOTEMAX | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 020583-001 | Mar 9, 1998 | 5,747,061*PED | ⤷ Try a Trial |
| Bausch And Lomb | PROLENSA | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 203168-001 | Apr 5, 2013 | 8,871,813 | ⤷ Try a Trial |
| Bausch And Lomb | PROLENSA | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 203168-001 | Apr 5, 2013 | 8,669,290 | ⤷ Try a Trial |
| Bausch And Lomb | RETISERT | fluocinolone acetonide | IMPLANT;INTRAVITREAL | 021737-001 | Apr 8, 2005 | 6,217,895 | ⤷ Try a Trial |
| Bausch And Lomb | VYZULTA | latanoprostene bunod | SOLUTION/DROPS;OPHTHALMIC | 207795-001 | Nov 2, 2017 | 6,211,233 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BAUSCH AND LOMB drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Ophthalmic Solution | 1.5% | ➤ Subscribe | 2013-09-09 |
| ➤ Subscribe | Ophthalmic Solution | 0.07% | ➤ Subscribe | 2013-07-26 |
| ➤ Subscribe | Ophthalmic Solution | 0.5% | ➤ Subscribe | 2012-10-19 |
International Patents for Bausch And Lomb Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 7082599 | ⤷ Try a Trial |
| Denmark | 2934510 | ⤷ Try a Trial |
| Canada | 3027767 | ⤷ Try a Trial |
| Japan | 6613342 | ⤷ Try a Trial |
| Eurasian Patent Organization | 032039 | ⤷ Try a Trial |
| China | 1902195 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bausch And Lomb Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0957929 | C 2007 038 | Romania | ⤷ Try a Trial | PRODUCT NAME: 5'-ESTERUL((2'-DEOXI-2'-FLUORO)C-GM-GM-A-A-(2'-DEOXI-2'-FLUORO)U-(2'-DEOXI-2'-FLUORO)C-AM-GM-(2'-DEOXI-2'-FLUORO)U-GM-AM-AM-(2'-DEOXI-2'-FLUORO)U-GM-(2'-DEOXI-2'-FLUORO)C-(2'-DEOXI-2'-FLUORO)U-(2'-DEOXI-2'-FLUORO)U-AM-(2'-DEOXI-2'-FLUORO)U-AM-(2'-DEOXI-2'-FLUORO)C-AM-(2'-DEOXI-2'-FLUORO)U-(2'-DEOXI-2'-FLUORO)C-(2'-DEOXI-2'-FLUORO)C-GM-(3'-3')-DT)CUALFA,ALFA'-[(1S)-1-[(5-(FOSFOONOXI)PENTIL]CARBAMOIL]PENTAN-1,5-DIIL]BIS(IMINOCARBONIL)]BIS[OMEGA-METOXIPOLI(OXIETAN-1,2-DIIL)], DE PREFERINTA SUB FORMA SARII DE SODIU - PEGAPTANIB, DE PREFERINTA SUB FORMA SARII DESODIU; NATIONAL AUTHORISATION NUMBER: EU/1/05/325/001; DATE OF NATIONAL AUTHORISATION: 20060131; NUMBER OF FIRST AUT [...] |
| 0694547 | 2002/028 | Ireland | ⤷ Try a Trial | PRODUCT NAME: VALGANCICLOVIR (2-(2-AMINO-1,6-DIHYDRO-6-OXO-PURIN-9-YL)- METHOXY-3-HYDROXY-1-PROPANYL-L-VALINATE) AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; NAT REGISTRATION NO/DATE: 50/150/1 20020913; FIRST REGISTRATION NO/DATE: NL 25992 20010920; PAEDIATRIC INVESTIGATION PLAN: P/0220/2013 |
| 1586316 | 1190018-0 | Sweden | ⤷ Try a Trial | RAETTELSE AV SKYDDSTID: 2024-01-17 TILL OCH MED DEN 2026-05-22 |
| 0364417 | SPC/GB97/014 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: LATANOPROST (I.E. 13,14-DIHYDRO-17-PHENYL-18,19,20-TRINOR-PGF-ALPHA-ISOPROPYLESTER); NAT. REGISTRATION NO/DATE: 00032/0220 19961216; FIRST REGISTRATION: SE 12716 19960718; SPC EXTENSION AUTHORISATION: PL00057/1057-008 20101216 |
| 1631293 | C300683 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: BRIMONIDINE EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN VOOR TOEPASSING ALS GENEESMIDDEL VOOR DE BEHANDELING VAN DOOR ROSACEA GEINDUCEERDE ROODHEID; REGISTRATION NO/DATE: EU/1/13/904 20140225 |
| 1631293 | 2014/041 | Ireland | ⤷ Try a Trial | PRODUCT NAME: BRIMONIDINE AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTRATION NO/DATE: EU/1/13/904 20140221 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.