BAYER HLTHCARE Company Profile
✉ Email this page to a colleague
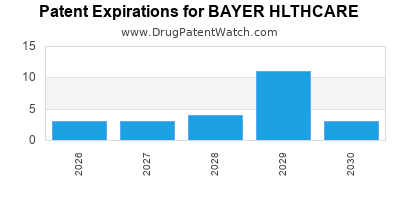
What is the competitive landscape for BAYER HLTHCARE, and when can generic versions of BAYER HLTHCARE drugs launch?
BAYER HLTHCARE has fifty-six approved drugs.
There are thirty-seven US patents protecting BAYER HLTHCARE drugs.
There are eight hundred and five patent family members on BAYER HLTHCARE drugs in fifty-eight countries and one hundred and forty-seven supplementary protection certificates in twenty countries.
Summary for BAYER HLTHCARE
| International Patents: | 805 |
| US Patents: | 37 |
| Tradenames: | 57 |
| Ingredients: | 37 |
| NDAs: | 56 |
Drugs and US Patents for BAYER HLTHCARE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | ADEMPAS | riociguat | TABLET;ORAL | 204819-001 | Oct 8, 2013 | AB | RX | Yes | No | 10,662,188 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Bayer Hlthcare | EOVIST | gadoxetate disodium | SOLUTION;INTRAVENOUS | 022090-001 | Jul 3, 2008 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bayer Hlthcare | ADEMPAS | riociguat | TABLET;ORAL | 204819-004 | Oct 8, 2013 | AB | RX | Yes | No | 11,203,593 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-002 | Jul 9, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bayer Hlthcare | VI-TWEL | cyanocobalamin | INJECTABLE;INJECTION | 007012-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bayer Hlthcare | VITRAKVI | larotrectinib sulfate | CAPSULE;ORAL | 210861-002 | Nov 26, 2018 | RX | Yes | Yes | 10,285,993 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BAYER HLTHCARE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | NEXAVAR | sorafenib tosylate | TABLET;ORAL | 021923-001 | Dec 20, 2005 | 7,897,623 | ⤷ Try a Trial |
| Bayer Hlthcare | YAZ | drospirenone; ethinyl estradiol | TABLET;ORAL | 021676-001 | Mar 16, 2006 | 7,163,931 | ⤷ Try a Trial |
| Bayer Hlthcare | AVELOX IN SODIUM CHLORIDE 0.8% IN PLASTIC CONTAINER | moxifloxacin hydrochloride | SOLUTION;INTRAVENOUS | 021277-001 | Nov 30, 2001 | 5,607,942 | ⤷ Try a Trial |
| Bayer Hlthcare | AVELOX | moxifloxacin hydrochloride | TABLET;ORAL | 021085-001 | Dec 10, 1999 | 5,607,942 | ⤷ Try a Trial |
| Bayer Hlthcare | CLIMARA | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 020375-001 | Dec 22, 1994 | 5,223,261 | ⤷ Try a Trial |
| Bayer Hlthcare | CLIMARA | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 020375-006 | May 27, 2003 | 5,223,261 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BAYER HLTHCARE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 0.5 mg, 1 mg,1.5 mg, 2 mg and2.5 mg | ➤ Subscribe | 2017-10-10 |
| ➤ Subscribe | Tablets | 0.25 mg/0.5 mg | ➤ Subscribe | 2015-01-08 |
| ➤ Subscribe | Tablets | 3 mg/0.02 mg/0.451 mg and 0.451 mg | ➤ Subscribe | 2012-11-13 |
| ➤ Subscribe | Tablets | 5 mg ad 10 mg | ➤ Subscribe | 2009-07-10 |
| ➤ Subscribe | Tablets | 3 mg; 2 mg/2 mg; 2 mg/3 mg; 1 mg | ➤ Subscribe | 2010-10-22 |
| ➤ Subscribe | Tablets | 25 mg, 50 mg and 100 mg | ➤ Subscribe | 2005-03-22 |
| ➤ Subscribe | Tablets | 40 mg | ➤ Subscribe | 2016-09-27 |
| ➤ Subscribe | Oral Suspension | 250 mg/5 mL and 500 mg/ 5 mL | ➤ Subscribe | 2009-10-16 |
| ➤ Subscribe | Tablets | 3 mg/0.03 mg | ➤ Subscribe | 2005-01-07 |
| ➤ Subscribe | Orally Disintegrating Tablets | 10 mg | ➤ Subscribe | 2011-12-22 |
| ➤ Subscribe | Tablets | 0.5 mg/1 mg | ➤ Subscribe | 2007-12-26 |
| ➤ Subscribe | Tablets | 3 mg/0.02 mg/0.451 mg and 0.451 mg | ➤ Subscribe | 2011-11-21 |
| ➤ Subscribe | Tablets | 20 mg | ➤ Subscribe | 2009-03-05 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2009-09-04 |
| ➤ Subscribe | Tablets | 200 mg | ➤ Subscribe | 2014-02-28 |
| ➤ Subscribe | Tablets | 3 mg/0.03 mg/0.451 mg and 0.451 mg | ➤ Subscribe | 2012-09-28 |
| ➤ Subscribe | Transdermal System | 0.05 mg/day and 0.1 mg/day | ➤ Subscribe | 2005-09-12 |
| ➤ Subscribe | Tablets | 3 mg/0.02 mg | ➤ Subscribe | 2006-09-29 |
| ➤ Subscribe | Injection | 1.6 mg/mL | ➤ Subscribe | 2014-02-07 |
International Patents for BAYER HLTHCARE Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 1946383 | ⤷ Try a Trial |
| Peru | 20180554 | ⤷ Try a Trial |
| Australia | 2012203343 | ⤷ Try a Trial |
| Lithuania | 3699181 | ⤷ Try a Trial |
| South Africa | 201100900 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2016077841 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for BAYER HLTHCARE Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | 122015000093 | Germany | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 87675.00.00 20150720; FIRST REGISTRATION: SLOWAKEI 17/0017/15-S 20150129 |
| 1663978 | C 2014 007 | Romania | ⤷ Try a Trial | PRODUCT NAME: REGORAFENIB SI SARURILE SALE4-[4-({[4-CLORO-3-(TRIFLUOROMETIL)-FENIL]CARBAMOIL}AMINO)-TIONAL AUTHORISATION: 20130826; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/858; DATE OF FIRST AUTHORISATION IN EEA: 20130826 3-FLUOROFENOXI]-N-METILPIRIDIN-2-CARBOXAMIDA; NATIONAL AUTHORISATION NUMBER: EU/1/13/858; DATE OF NA |
| 1453521 | 39/2015 | Austria | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL UND EINE KOMBINATION VON LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 136021 20150224; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 3106463 | C20200005 00308 | Estonia | ⤷ Try a Trial | PRODUCT NAME: LAROTREKTINIIB;REG NO/DATE: EU/1/19/1385; 23.09.2019 |
| 3106463 | PA2020504,C3106463 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: LAROTREKTINIBAS IR (ARBA) FARMACINIU POZIURIU PRIIMTINOS DRUSKOS, YPAC LAROTREKTINIBO SULFATAS, ISKAITANT LAROTREKTINIBO VANDENILIO SULFATA; REGISTRATION NO/DATE: EU/1/19/1385 20190919 |
| 3632448 | 22C1031 | France | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE; NAT. REGISTRATION NO/DATE: NL49691 20191121; FIRST REGISTRATION: DK - 31332 20191016 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.