Astrazeneca Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ASTRAZENECA, and when can generic versions of ASTRAZENECA drugs launch?
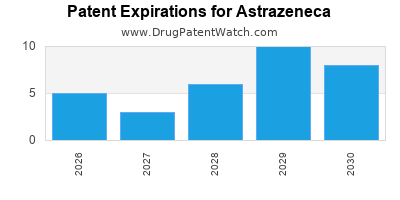
ASTRAZENECA has ninety-three approved drugs.
There are one hundred and twenty-four US patents protecting ASTRAZENECA drugs. There is one tentative approval on ASTRAZENECA drugs.
There are two thousand one hundred and ninety-two patent family members on ASTRAZENECA drugs in seventy countries and three hundred and forty-six supplementary protection certificates in twenty countries.
Summary for Astrazeneca
| International Patents: | 2192 |
| US Patents: | 124 |
| Tradenames: | 66 |
| Ingredients: | 60 |
| NDAs: | 93 |
| Patent Litigation for Astrazeneca: | See patent lawsuits for Astrazeneca |
| PTAB Cases with Astrazeneca as patent owner: | See PTAB cases with Astrazeneca as patent owner |
Drugs and US Patents for Astrazeneca
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | LYNPARZA | olaparib | TABLET;ORAL | 208558-001 | Aug 17, 2017 | RX | Yes | No | 8,143,241 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Astrazeneca | SEROQUEL XR | quetiapine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022047-002 | May 17, 2007 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-001 | Feb 27, 2017 | RX | Yes | Yes | 8,628,799 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-001 | Feb 28, 2011 | AB | RX | Yes | Yes | 8,618,142 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Astrazeneca | TRUQAP | capivasertib | TABLET;ORAL | 218197-001 | Nov 16, 2023 | RX | Yes | No | 8,809,336 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Astrazeneca
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | NEXIUM | esomeprazole magnesium | CAPSULE, DELAYED REL PELLETS;ORAL | 021153-002 | Feb 20, 2001 | 4,508,905 | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-001 | Feb 27, 2017 | RE44186 | ⤷ Try a Trial |
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-001 | Mar 16, 2005 | 7,407,934 | ⤷ Try a Trial |
| Astrazeneca | ELAVIL | amitriptyline hydrochloride | TABLET;ORAL | 012703-007 | Approved Prior to Jan 1, 1982 | 3,428,735 | ⤷ Try a Trial |
| Astrazeneca | LEXXEL | enalapril maleate; felodipine | TABLET, EXTENDED RELEASE;ORAL | 020668-001 | Dec 27, 1996 | 4,803,081*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ASTRAZENECA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Capsules | 20 mg | ➤ Subscribe | 2007-03-19 |
| ➤ Subscribe | Extended-release Tablets | 400 mg | ➤ Subscribe | 2008-06-18 |
| ➤ Subscribe | Tablets | 200 mg and 300 mg | ➤ Subscribe | 2008-06-12 |
| ➤ Subscribe | Tablets | 90 mg | ➤ Subscribe | 2015-07-20 |
| ➤ Subscribe | Tablets | 500 mcg | ➤ Subscribe | 2015-03-02 |
| ➤ Subscribe | Nasal Spray | 0.032 mg (32 mcg)/spray | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | Tablets | 50 mg, 150 mg and 400 mg | ➤ Subscribe | 2007-02-12 |
| ➤ Subscribe | Delayed-release for Oral Suspension | 2.5 mg and 5 mg | ➤ Subscribe | 2018-09-24 |
| ➤ Subscribe | Injection | 250 mg/mL, 1.2 mL and 2.4 mL prefilled syringe | ➤ Subscribe | 2014-06-11 |
| ➤ Subscribe | Injection | 50 mg/mL, 2.5 mL and 5 mL syringe | ➤ Subscribe | 2009-10-01 |
| ➤ Subscribe | HydrochlorideExtended-release Tablets | 2.5 mg/1000 mg | ➤ Subscribe | 2018-10-29 |
| ➤ Subscribe | Inhalation Suspension | 1 mg/2 mL | ➤ Subscribe | 2010-05-28 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2012-03-30 |
| ➤ Subscribe | Extended-release Tablets | 150 mg | ➤ Subscribe | 2008-11-17 |
| ➤ Subscribe | Extended-release Tablets | 50 mg | ➤ Subscribe | 2008-10-17 |
| ➤ Subscribe | Tablets | 60 mg | ➤ Subscribe | 2015-09-30 |
| ➤ Subscribe | Tablets | 25 mg | ➤ Subscribe | 2005-08-12 |
| ➤ Subscribe | Delayed-release | 20 mg and 40 mg | ➤ Subscribe | 2005-08-05 |
| ➤ Subscribe | Tablets | 100 mg, 200 mg and 300 mg | ➤ Subscribe | 2006-02-21 |
| ➤ Subscribe | For Injection | 20 mg/vial and 40 mg/vial | ➤ Subscribe | 2009-11-23 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/500 mg, 2.5 mg/1000 mg, and 5 mg/1000 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Inhalation Suspension | 0.25 mg/2 mL and 0.5 mg/2 mL | ➤ Subscribe | 2005-09-15 |
| ➤ Subscribe | Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2013-07-31 |
International Patents for Astrazeneca Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Croatia | P20041084 | ⤷ Try a Trial |
| Russian Federation | 2755865 | ⤷ Try a Trial |
| Ukraine | 120287 | ⤷ Try a Trial |
| Japan | 5873013 | ⤷ Try a Trial |
| Belgium | 2015C024 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Astrazeneca Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2139494 | CA 2020 00035 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN OG DAPAGLIFLOZIN; REG. NO/DATE: EU/1/16/1108 20160719 |
| 2435024 | 301102 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN FORMOTEROL (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN), GLYCOPYRROLAAT (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN) EN BUDESONIDE (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN); REGISTRATION NO/DATE: EU/1/20/1498 20201210 |
| 1506211 | 1390017-0 | Sweden | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZIN OCH FARMACEUTISKT GODTAGBARA SALTER DAERAV; REG. NO/DATE: EU/1/12/795/001 20121112 |
| 1734971 | C 2012 010 | Romania | ⤷ Try a Trial | PRODUCT NAME: EXENATIDA; NATIONAL AUTHORISATION NUMBER: EU/1/11/696/001, EU/1/11/696/002; DATE OF NATIONAL AUTHORISATION: 20110623; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EMEA EU/1/11/696/001, EMEA EU/1/11/696/002; DATE OF FIRST AUTHORISATION IN EEA: 20110623 |
| 2498758 | 301040 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: METFORMINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; SAXAGLIPTINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; DAPAGLIFLOZINE OF EEN FARMACEUTISCH AANVAARDBAAR SOLVAAT DAARVAN; REGISTRATION NO/DATE: EU/1/19/1401 20191113 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.