Abbvie Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ABBVIE, and what generic alternatives to ABBVIE drugs are available?
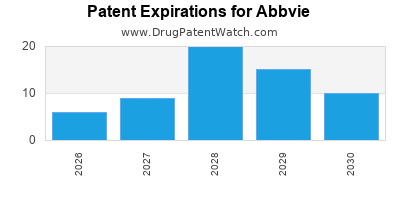
ABBVIE has one hundred and thirty-eight approved drugs.
There are two hundred and twenty-nine US patents protecting ABBVIE drugs.
There are two thousand seven hundred and twenty-one patent family members on ABBVIE drugs in sixty-six countries and three hundred and ninety-one supplementary protection certificates in nineteen countries.
Summary for Abbvie
| International Patents: | 2721 |
| US Patents: | 229 |
| Tradenames: | 124 |
| Ingredients: | 99 |
| NDAs: | 138 |
| Drug Master File Entries: | 1 |
| Patent Litigation for Abbvie: | See patent lawsuits for Abbvie |
Drugs and US Patents for Abbvie
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | SYNTHROID | levothyroxine sodium | TABLET;ORAL | 021402-006 | Jul 24, 2002 | AB1,AB2 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | ORIAHNN (COPACKAGED) | elagolix sodium,estradiol,norethindrone acetate; elagolix sodium | CAPSULE;ORAL | 213388-001 | May 29, 2020 | RX | Yes | Yes | 11,542,239 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Abbvie | VRAYLAR | cariprazine hydrochloride | CAPSULE;ORAL | 204370-003 | Sep 17, 2015 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Abbvie
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-004 | Jul 28, 1997 | 6,129,930 | ⤷ Try a Trial |
| Abbvie | OZURDEX | dexamethasone | IMPLANT;INTRAVITREAL | 022315-001 | Jun 17, 2009 | 9,592,242 | ⤷ Try a Trial |
| Abbvie | CARAFATE | sucralfate | TABLET;ORAL | 018333-001 | Approved Prior to Jan 1, 1982 | 3,432,489 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ABBVIE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 100 mg/25 mg | ➤ Subscribe | 2008-12-23 |
| ➤ Subscribe | Extended-release Tablets | 7.5 mg and 15 mg | ➤ Subscribe | 2008-12-22 |
| ➤ Subscribe | Ophthalmic Solution | 0.15% | ➤ Subscribe | 2006-11-03 |
| ➤ Subscribe | Capsules | 145 mcg and 290 mcg | ➤ Subscribe | 2016-08-30 |
| ➤ Subscribe | Extended-release Tablets | 1 mg/240 mg | ➤ Subscribe | 2008-02-20 |
| ➤ Subscribe | Ophthalmic Solution | 0.25% | ➤ Subscribe | 2014-07-30 |
| ➤ Subscribe | Capsules | 100 mg | ➤ Subscribe | 2012-10-31 |
| ➤ Subscribe | Tablets | 5 mg/80 mg | ➤ Subscribe | 2017-06-09 |
| ➤ Subscribe | Ophthalmic Solution | 0.01% | ➤ Subscribe | 2011-04-05 |
| ➤ Subscribe | Delayed-release Capsules | 400 mg | ➤ Subscribe | 2014-06-17 |
| ➤ Subscribe | Capsules | 1 mcg and 2 mcg | ➤ Subscribe | 2008-10-14 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2007-10-16 |
| ➤ Subscribe | Topical Solution | 0.03% | ➤ Subscribe | 2010-05-03 |
| ➤ Subscribe | Delayed-release Capsules | 135 mg | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Tablets | 400 mg and 600 mg | ➤ Subscribe | 2010-05-10 |
| ➤ Subscribe | Gel | 7.5% | ➤ Subscribe | 2017-02-13 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg, and 28 mg | ➤ Subscribe | 2013-08-16 |
| ➤ Subscribe | Capsules | 10 mg and 20 mg | ➤ Subscribe | 2005-03-30 |
| ➤ Subscribe | Tablets | 145 mg | ➤ Subscribe | 2007-10-19 |
| ➤ Subscribe | Injection | 2 mg/mL, 5 mL vial and 10 mg/mL, 20 mL vial | ➤ Subscribe | 2009-08-04 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg | ➤ Subscribe | 2013-06-17 |
| ➤ Subscribe | Suppository | 1000 mg | ➤ Subscribe | 2013-05-24 |
| ➤ Subscribe | Tablets | 5 mg/200 mg | ➤ Subscribe | 2005-05-27 |
| ➤ Subscribe | Injection | 400 mg/vial and 600 mg/vial | ➤ Subscribe | 2014-10-29 |
| ➤ Subscribe | Capsules | 14 mg/10 mg and 28 mg/10 mg | ➤ Subscribe | 2015-05-18 |
| ➤ Subscribe | Extended-release Tablet | 500 mg | ➤ Subscribe | 2010-12-06 |
| ➤ Subscribe | Opthalmic Solution | 0.45% | ➤ Subscribe | 2011-08-23 |
| ➤ Subscribe | Capsules | 7 mg/10 mg | ➤ Subscribe | 2016-09-26 |
| ➤ Subscribe | Extended-release Tablets | 500 mg | ➤ Subscribe | 2005-02-08 |
| ➤ Subscribe | Ophthalmic Solution | 0.10% | ➤ Subscribe | 2006-12-20 |
| ➤ Subscribe | Oral Solution | 80 mg/20 mg per mL | ➤ Subscribe | 2014-06-19 |
| ➤ Subscribe | Extended-release Tablets | 2 mg/180 mg and 2 mg/240 mg | ➤ Subscribe | 2007-11-09 |
| ➤ Subscribe | Ophthalmic Solution | 0.2%/0.5% | ➤ Subscribe | 2008-11-21 |
| ➤ Subscribe | Tablets | 100 mg | ➤ Subscribe | 2010-12-21 |
| ➤ Subscribe | Extended-release Tablets | 4 mg/ 240 mg | ➤ Subscribe | 2007-07-24 |
| ➤ Subscribe | Ophthalmic Solution | 0.03% | ➤ Subscribe | 2008-12-22 |
| ➤ Subscribe | Injection | 0.002 mg per mL in 1 mL vial and 0.005 mg per mL in 1 mL and 2 mL vials | ➤ Subscribe | 2008-11-28 |
| ➤ Subscribe | Tablets | 1 mg, 2 mg and 4 mg | ➤ Subscribe | 2004-10-04 |
| ➤ Subscribe | Ophthalmic Solution | 0.5% | ➤ Subscribe | 2010-12-07 |
| ➤ Subscribe | Delayed-release Capsules | 45 mg | ➤ Subscribe | 2009-09-02 |
| ➤ Subscribe | Tablets | 12.5 mg, 25 mg, 50 mg, and 100 mg | ➤ Subscribe | 2013-01-14 |
| ➤ Subscribe | Capsules | 4 mcg | ➤ Subscribe | 2008-08-25 |
| ➤ Subscribe | Capsules | 5 mg | ➤ Subscribe | 2005-08-17 |
| ➤ Subscribe | Extended-release Capsules | 20 mg, 40 mg, 80 mg and 120 mg | ➤ Subscribe | 2017-07-25 |
| ➤ Subscribe | Tablets | 48 mg | ➤ Subscribe | 2008-07-01 |
| ➤ Subscribe | Gel | 10% | ➤ Subscribe | 2014-06-19 |
| ➤ Subscribe | Extended-release Capsules | 28 mg | ➤ Subscribe | 2013-06-12 |
| ➤ Subscribe | Injection | 10 mg/mL (2 mL) | ➤ Subscribe | 2018-07-13 |
| ➤ Subscribe | Tablets | 2.5 mg/200 mg | ➤ Subscribe | 2006-02-24 |
| ➤ Subscribe | Injection | 2 mg/mL, 10 mL vial | ➤ Subscribe | 2009-08-12 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg, and 28 mg | ➤ Subscribe | 2013-06-10 |
| ➤ Subscribe | Delayed-release Tablets | 800 mg | ➤ Subscribe | 2011-07-13 |
| ➤ Subscribe | Tablets | 10 mg, 20 mg, and 40 mg | ➤ Subscribe | 2015-01-21 |
| ➤ Subscribe | Ophthalmic Solution | 0.40% | ➤ Subscribe | 2005-01-28 |
| ➤ Subscribe | Capsules | 21 mg/10 mg | ➤ Subscribe | 2016-09-23 |
| ➤ Subscribe | Extended-release Tablets | 250 mg | ➤ Subscribe | 2004-05-03 |
| ➤ Subscribe | Opthalmic Solution | 0.45% | ➤ Subscribe | 2011-08-24 |
International Patents for Abbvie Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Costa Rica | 11260 | ⤷ Try a Trial |
| Poland | 2643322 | ⤷ Try a Trial |
| Canada | 2736895 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Abbvie Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2203431 | 1590018-6 | Sweden | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REG. NO/DATE: EU/1/14/983 20150119 |
| 2203431 | 2015/009 | Ireland | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REGISTRATION NO/DATE: EU/1/14/983 20150115 |
| 0473687 | SPC/GB98/030 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FOSPHENYTOIN SODIUM (3-HYDROXYMETHYL)-5,5-DIPHENYLHYDANTOIN DISODIUM PHOSPHATE ESTER); REGISTERED: UK 0019/0157 19980204 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.