ONGLYZA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Onglyza, and what generic alternatives are available?
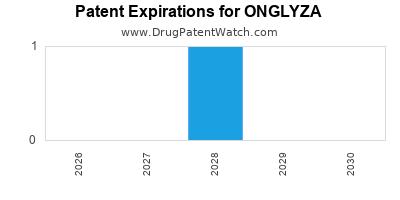
Onglyza is a drug marketed by Astrazeneca Ab and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has fifty-seven patent family members in thirty-two countries.
The generic ingredient in ONGLYZA is saxagliptin hydrochloride. There are fifteen drug master file entries for this compound. Six suppliers are listed for this compound. Additional details are available on the saxagliptin hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Onglyza
Onglyza was eligible for patent challenges on July 31, 2013.
There have been sixteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (saxagliptin hydrochloride), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for ONGLYZA
| International Patents: | 57 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 134 |
| Clinical Trials: | 68 |
| Patent Applications: | 4,141 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ONGLYZA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ONGLYZA |
| What excipients (inactive ingredients) are in ONGLYZA? | ONGLYZA excipients list |
| DailyMed Link: | ONGLYZA at DailyMed |

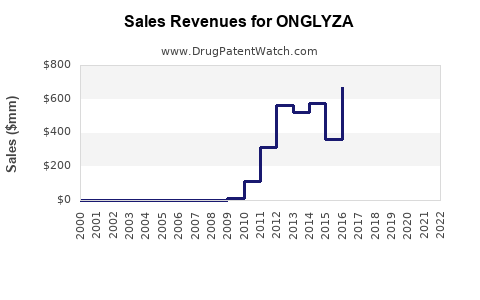
Recent Clinical Trials for ONGLYZA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Ain Shams University | N/A |
| Beni-Suef University | Phase 4 |
| Sabyasachi Sen | Phase 4 |
Pharmacology for ONGLYZA
| Drug Class | Dipeptidyl Peptidase 4 Inhibitor |
| Mechanism of Action | Dipeptidyl Peptidase 4 Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ONGLYZA
Paragraph IV (Patent) Challenges for ONGLYZA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ONGLYZA | Tablets | saxagliptin hydrochloride | 2.5 mg and 5 mg | 022350 | 8 | 2013-07-31 |
US Patents and Regulatory Information for ONGLYZA
ONGLYZA is protected by one US patents.
Patents protecting ONGLYZA
Coated tablet formulation and method
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | ONGLYZA | saxagliptin hydrochloride | TABLET;ORAL | 022350-001 | Jul 31, 2009 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | ONGLYZA | saxagliptin hydrochloride | TABLET;ORAL | 022350-002 | Jul 31, 2009 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ONGLYZA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | ONGLYZA | saxagliptin hydrochloride | TABLET;ORAL | 022350-002 | Jul 31, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | ONGLYZA | saxagliptin hydrochloride | TABLET;ORAL | 022350-002 | Jul 31, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | ONGLYZA | saxagliptin hydrochloride | TABLET;ORAL | 022350-001 | Jul 31, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | ONGLYZA | saxagliptin hydrochloride | TABLET;ORAL | 022350-001 | Jul 31, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ONGLYZA
When does loss-of-exclusivity occur for ONGLYZA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 9062
Estimated Expiration: ⤷ Try a Trial
Patent: 9567
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 05249467
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0510419
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 68391
Estimated Expiration: ⤷ Try a Trial
China
Patent: 88891
Estimated Expiration: ⤷ Try a Trial
Patent: 2895208
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0160880
Estimated Expiration: ⤷ Try a Trial
Patent: 0161210
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 17813
Estimated Expiration: ⤷ Try a Trial
Patent: 18162
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 53406
Estimated Expiration: ⤷ Try a Trial
Patent: 98288
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 53406
Estimated Expiration: ⤷ Try a Trial
Patent: 98288
Estimated Expiration: ⤷ Try a Trial
Patent: 78369
Estimated Expiration: ⤷ Try a Trial
Georgia, Republic of
Patent: 0094639
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 94951
Estimated Expiration: ⤷ Try a Trial
Patent: 55399
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 29039
Estimated Expiration: ⤷ Try a Trial
Patent: 29446
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 9454
Estimated Expiration: ⤷ Try a Trial
Patent: 8117
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 01727
Estimated Expiration: ⤷ Try a Trial
Patent: 08501025
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 98288
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 7639
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 06013711
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 516
Estimated Expiration: ⤷ Try a Trial
Patent: 643
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 1591
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 3907
Estimated Expiration: ⤷ Try a Trial
Patent: 065870
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 060425
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 53406
Estimated Expiration: ⤷ Try a Trial
Patent: 98288
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 53406
Estimated Expiration: ⤷ Try a Trial
Patent: 98288
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 72894
Estimated Expiration: ⤷ Try a Trial
Patent: 06146971
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 929
Estimated Expiration: ⤷ Try a Trial
Patent: 174
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 53406
Estimated Expiration: ⤷ Try a Trial
Patent: 98288
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0609541
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1290925
Estimated Expiration: ⤷ Try a Trial
Patent: 070027560
Estimated Expiration: ⤷ Try a Trial
Patent: 120064141
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 82646
Estimated Expiration: ⤷ Try a Trial
Patent: 93582
Estimated Expiration: ⤷ Try a Trial
Patent: 54573
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 54569
Estimated Expiration: ⤷ Try a Trial
Patent: 15635
Estimated Expiration: ⤷ Try a Trial
Patent: 0609002
Estimated Expiration: ⤷ Try a Trial
Patent: 1204414
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 168
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ONGLYZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2593582 | ⤷ Try a Trial | |
| China | 1213028 | ⤷ Try a Trial | |
| South Korea | 101290925 | ⤷ Try a Trial | |
| South Korea | 100754089 | ⤷ Try a Trial | |
| Montenegro | 02643 | FORMULACIJA I METOD OBLOŽENE TABLETE (COATED T ABLET FORMULATION AND METHOD) | ⤷ Try a Trial |
| Serbia | 54929 | FORMULACIJA I METOD OBLOŽENE TABLETE (COATED TABLET FORMULATION AND METHOD) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ONGLYZA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1261586 | C01261586/01 | Switzerland | ⤷ Try a Trial | FORMER OWNER: BRISTOL-MYERS SQUIBB COMPANY, US |
| 2498758 | 2090013-0 | Sweden | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF METFORMIN HYDROCHLORIDE, SAXAGLIPTIN OR A PHARMACEUTICALLY ACCEPABLE SALT THEREOF, AND DAPAGLIFLOZIN OR PH ARMACEUTICALLY ACCEPTABLE SOLVATE THEREOF; REG. NO/DATE: EU/1/19/1401 20191113 |
| 2139494 | CA 2020 00035 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN OG DAPAGLIFLOZIN; REG. NO/DATE: EU/1/16/1108 20160719 |
| 1261586 | 2010C/008 | Belgium | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTINE ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES, COMPRENANT LE CHLORHYDRATE DE SAXAGLIPTINE; AUTHORISATION NUMBER AND DATE: EU/1/09/545001 20091005 |
| 2139494 | 301054 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN AND DAPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/16/1108 20160719 |
| 1261586 | SZ 4/2010 | Austria | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN UND PHARMAZEUTISCH VERTRAEGLICHE SALZE DAVON, BEINHALTEND SAXAGLIPTINHYDROCHLORID |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


