DIGOXIN Drug Patent Profile
✉ Email this page to a colleague
When do Digoxin patents expire, and what generic alternatives are available?
Digoxin is a drug marketed by Amici, Hikma, Vistapharm Llc, Abraxis Pharm, Hospira, Sandoz, West-ward Pharms Int, Wyeth Ayerst, Aurobindo Pharma Ltd, Hikma Intl Pharms, Impax Labs, Novitium Pharma, Rising, Stevens J, and Sun Pharm Inds Inc. and is included in seventeen NDAs.
The generic ingredient in DIGOXIN is digoxin. There are ten drug master file entries for this compound. Twenty-eight suppliers are listed for this compound. Additional details are available on the digoxin profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Digoxin
A generic version of DIGOXIN was approved as digoxin by RISING on December 23rd, 1999.
Summary for DIGOXIN
| US Patents: | 0 |
| Applicants: | 15 |
| NDAs: | 17 |
| Finished Product Suppliers / Packagers: | 25 |
| Raw Ingredient (Bulk) Api Vendors: | 87 |
| Clinical Trials: | 224 |
| Patent Applications: | 3,841 |
| Formulation / Manufacturing: | see details |
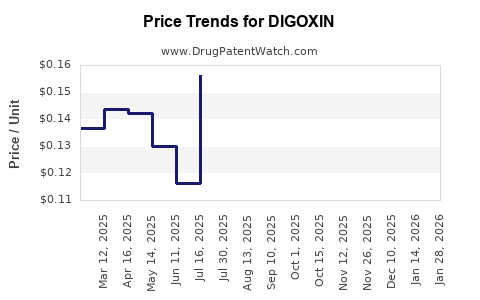
| Drug Prices: | Drug price information for DIGOXIN |
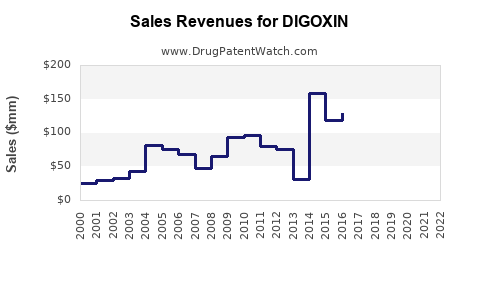
| Drug Sales Revenues: | Drug sales revenues for DIGOXIN |
| What excipients (inactive ingredients) are in DIGOXIN? | DIGOXIN excipients list |
| DailyMed Link: | DIGOXIN at DailyMed |
Recent Clinical Trials for DIGOXIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Innovent Biologics (Suzhou) Co. Ltd. | Phase 1 |
| Boehringer Ingelheim | N/A |
| AbbVie | Phase 1 |
Pharmacology for DIGOXIN
| Drug Class | Cardiac Glycoside |
Medical Subject Heading (MeSH) Categories for DIGOXIN
Anatomical Therapeutic Chemical (ATC) Classes for DIGOXIN
US Patents and Regulatory Information for DIGOXIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amici | DIGOXIN | digoxin | ELIXIR;ORAL | 215209-001 | Mar 11, 2022 | AA | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Novitium Pharma | DIGOXIN | digoxin | TABLET;ORAL | 215307-001 | Nov 22, 2021 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Sun Pharm Inds Inc | DIGOXIN | digoxin | TABLET;ORAL | 076363-001 | Jan 31, 2003 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Sandoz | DIGOXIN | digoxin | INJECTABLE;INJECTION | 040481-001 | Aug 21, 2003 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


