SODIUM CHLORIDE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Sodium Chloride, and what generic alternatives are available?
Sodium Chloride is a drug marketed by Abbott, B Braun, Baxter Hlthcare, Fresenius Kabi Usa, Hospira, Icu Medical Inc, Miles, Medefil Inc, Hikma, Nexus Pharms, Spectra Mdcl Devices, Fresenius Medcl, Haemonetics, Laboratorios Grifols, Liebel-flarsheim, Jubilant Cadista, Nephron, Taro, and Abraxis Pharm. and is included in fifty-three NDAs.
The generic ingredient in SODIUM CHLORIDE is potassium chloride; sodium chloride. There are two hundred and forty drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the potassium chloride; sodium chloride profile page.
Summary for SODIUM CHLORIDE
| US Patents: | 0 |
| Applicants: | 19 |
| NDAs: | 53 |
| Raw Ingredient (Bulk) Api Vendors: | 328 |
| Patent Applications: | 3,030 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for SODIUM CHLORIDE |
| What excipients (inactive ingredients) are in SODIUM CHLORIDE? | SODIUM CHLORIDE excipients list |
| DailyMed Link: | SODIUM CHLORIDE at DailyMed |
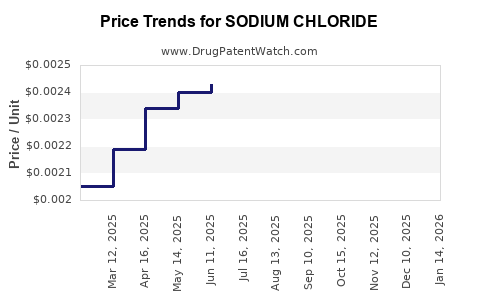
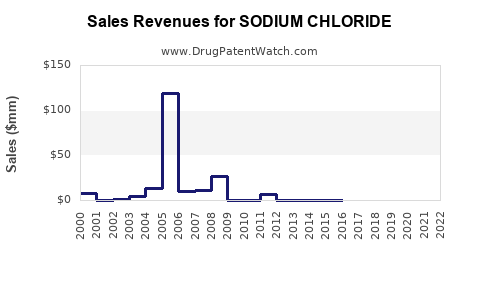
See drug prices for SODIUM CHLORIDE
Anatomical Therapeutic Chemical (ATC) Classes for SODIUM CHLORIDE
US Patents and Regulatory Information for SODIUM CHLORIDE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taro | SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | sodium chloride | SOLUTION;INJECTION | 077407-002 | Aug 11, 2006 | AP | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Liebel-flarsheim | SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | sodium chloride | INJECTABLE;INJECTION | 021569-002 | Jul 27, 2006 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| B Braun | SODIUM CHLORIDE 0.9% AND POTASSIUM CHLORIDE 0.3% IN PLASTIC CONTAINER | potassium chloride; sodium chloride | INJECTABLE;INJECTION | 018722-004 | Nov 9, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Fresenius Kabi Usa | SODIUM CHLORIDE 23.4% | sodium chloride | SOLUTION;INTRAVENOUS | 212070-003 | Feb 14, 2022 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Baxter Hlthcare | SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | sodium chloride | SOLUTION;INJECTION | 020178-001 | Dec 7, 1992 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Hospira | SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | sodium chloride | SOLUTION;INJECTION | 019465-002 | Jul 15, 1985 | AP | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Baxter Hlthcare | SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | sodium chloride | SOLUTION;INJECTION | 016677-004 | Oct 30, 1985 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


