PREZISTA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Prezista, and what generic alternatives are available?
Prezista is a drug marketed by Janssen Prods and is included in two NDAs. There are two patents protecting this drug and two Paragraph IV challenges.
This drug has forty-eight patent family members in twenty-five countries.
The generic ingredient in PREZISTA is darunavir. There are twenty-five drug master file entries for this compound. Ten suppliers are listed for this compound. Additional details are available on the darunavir profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Prezista
A generic version of PREZISTA was approved as darunavir by TEVA PHARMS USA on November 21st, 2017.
Summary for PREZISTA
| International Patents: | 48 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 96 |
| Clinical Trials: | 44 |
| Patent Applications: | 5,940 |
| Formulation / Manufacturing: | see details |
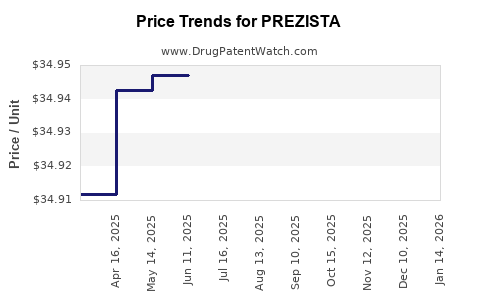
| Drug Prices: | Drug price information for PREZISTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PREZISTA |
| What excipients (inactive ingredients) are in PREZISTA? | PREZISTA excipients list |
| DailyMed Link: | PREZISTA at DailyMed |
Recent Clinical Trials for PREZISTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Frederick National Laboratory for Cancer Research | Phase 4 |
| Geropharm | N/A |
| Wits Reproductive Health and HIV Institute | Phase 1 |
Pharmacology for PREZISTA
| Drug Class | Protease Inhibitor |
| Mechanism of Action | Cytochrome P450 2D6 Inhibitors Cytochrome P450 3A Inhibitors HIV Protease Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for PREZISTA
US Patents and Regulatory Information for PREZISTA
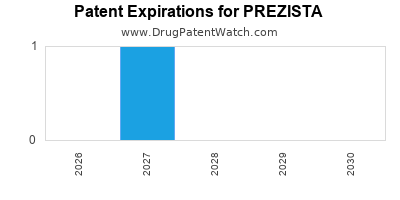
PREZISTA is protected by two US patents.
Patents protecting PREZISTA
Pseudopolymorphic forms of a HIV protease inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pseudopolymorphic forms of a HIV protease inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Prods | PREZISTA | darunavir | SUSPENSION;ORAL | 202895-001 | Dec 16, 2011 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-001 | Jun 23, 2006 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-004 | Dec 18, 2008 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-002 | Feb 25, 2008 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-001 | Jun 23, 2006 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-006 | Nov 9, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PREZISTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-002 | Feb 25, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-006 | Nov 9, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-001 | Jun 23, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-005 | Dec 18, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-003 | Oct 21, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-003 | Oct 21, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for PREZISTA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Mylan Pharmaceuticals Limited | Darunavir Mylan | darunavir | EMEA/H/C/004068 Darunavir, co-administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV-1) infection (see section 4.2).Darunavir Mylan 75 mg, 150 mg, 300 mg and 600 mg tablets may be used to provide suitable dose regimens (see section 4.2):For the treatment of HIV-1 infection in antiretroviral treatment (ART)-experienced adult patients, including those that have been highly pre-treated.For the treatment of HIV-1 infection in paediatric patients from the age of 3 years and at least 15 kg body weight.In deciding to initiate treatment with darunavir co-administered with low dose ritonavir, careful consideration should be given to the treatment history of the individual patient and the patterns of mutations associated with different agents. Genotypic or phenotypic testing (when available) and treatment history should guide the use of darunavir (see sections 4.2, 4.4 and 5.1).Darunavir co-administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV-1) infection. Darunavir co-administered with cobicistat is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus (HIV-1) infection in adults and adolescents (aged 12 years and older, weighing at least 40 kg) (see section 4.2). Darunavir Mylan 400 mg and 800 mg tablets may be used to provide suitable dose regimens for the treatment of HIV-1 infection in adult and paediatric patients from the age of 3 years and at least 40 kg body weight who are: antiretroviral therapy (ART)-naïve (see section 4.2). ART-experienced with no darunavir resistance associated mutations (DRV-RAMs) and who have plasma HIV-1 RNA < 100,000 copies/ml and CD4+ cell count ≥ 100 cells x 10⁶/L. In deciding to initiate treatment with darunavir in such ART-experienced patients, genotypic testing should guide the use of darunavir (see sections 4.2, 4.3, 4.4 and 5.1). |
Authorised | yes | no | no | 2017-01-03 | |
| Janssen-Cilag International NV | Prezista | darunavir | EMEA/H/C/000707 PREZISTA, co administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus (HIV 1) infection in adult and paediatric patients from the age of 3 years and at least 15 kg body weight.PREZISTA, co administered with cobicistat is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus (HIV 1) infection in adults and adolescents (aged 12 years and older, weighing at least 40 kg).In deciding to initiate treatment with PREZISTA co administered with cobicistat or low dose ritonavir, careful consideration should be given to the treatment history of the individual patient and the patterns of mutations associated with different agents. Genotypic or phenotypic testing (when available) and treatment history should guide the use of PREZISTA.PREZISTA, co administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV 1) infection.PREZISTA 75 mg, 150 mg, and 600 mg tablets may be used to provide suitable dose regimens:For the treatment of HIV 1 infection in antiretroviral treatment (ART) experienced adult patients, including those that have been highly pre treated.For the treatment of HIV 1 infection in paediatric patients from the age of 3 years and at least 15 kg body weight.In deciding to initiate treatment with PREZISTA co administered with low dose ritonavir, careful consideration should be given to the treatment history of the individual patient and the patterns of mutations associated with different agents. Genotypic or phenotypic testing (when available) and treatment history should guide the use of PREZISTA.PREZISTA, co administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV 1) infection.PREZISTA, co administered with cobicistat is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus (HIV 1) infection in adults and adolescents (aged 12 years and older, weighing at least 40 kg).PREZISTA 400 mg and 800 mg tablets may be used to provide suitable dose regimens for the treatment of HIV 1 infection in adult and paediatric patients from the age of 3 years and at least 40 kg body weight who are:antiretroviral therapy (ART) naïve.ART experienced with no darunavir resistance associated mutations (DRV RAMs) and who have plasma HIV 1 RNA < 100,000 copies/ml and CD4+ cell count ≥ 100 cells x 106/L. In deciding to initiate treatment with PREZISTA in such ART experienced patients, genotypic testing should guide the use of PREZISTA. |
Authorised | no | no | no | 2007-02-11 | |
| KRKA, d.d., Novo mesto | Darunavir Krka d.d. | darunavir | EMEA/H/C/004891 400mg and 800 mg Film-coated TabletsDarunavir Krka d.d., co-administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV-1) infection.Darunavir Krka d.d., co-administered with cobicistat is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV-1) infection in adult patients (see section 4.2).Darunavir Krka d.d. 400 mg and 800 mg tablets may be used to provide suitable dose regimens for the treatment of HIV-1 infection in adult and paediatric patients from the age of 3 years and at least 40 kg body weight who are:antiretroviral therapy (ART)-naïve (see section 4.2).ART-experienced with no darunavir resistance associated mutations (DRV-RAMs) and who have plasma HIV-1 RNA < 100,000 copies/ml and CD4+ cell count ≥ 100 cells x 106/l. In deciding to initiate treatment with darunavir in such ART-experienced patients, genotypic testing should guide the use of darunavir (see sections 4.2, 4.3, 4.4 and 5.1).600mg Film-coated TabletsDarunavir Krka d.d., co-administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV-1) infection.Darunavir Krka d.d. 600 mg tablets may be used to provide suitable dose regimens (see section 4.2):For the treatment of HIV-1 infection in antiretroviral treatment (ART)-experienced adult patients, including those that have been highly pre-treated.For the treatment of HIV-1 infection in paediatric patients from the age of 3 years and at least 15 kg body weight.In deciding to initiate treatment with darunavir co-administered with low dose ritonavir, careful consideration should be given to the treatment history of the individual patient and the patterns of mutations associated with different agents. Genotypic or phenotypic testing (when available) and treatment history should guide the use of darunavir. |
Withdrawn | yes | no | no | 2018-01-18 | |
| KRKA, d.d., Novo mesto | Darunavir Krka | darunavir | EMEA/H/C/004273 400 and 800 mgDarunavir Krka, co-administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV-1) infection.Darunavir Krka 400 mg and 800 mg tablets may be used to provide suitable dose regimens for the treatment of HIV-1 infection in adult and paediatric patients from the age of 3 years and at least 40 kg body weight who are:antiretroviral therapy (ART)-naïve (see section 4.2).ART-experienced with no darunavir resistance associated mutations (DRV-RAMs) and who have plasma HIV-1 RNA < 100,000 copies/ml and CD4+ cell count ≥ 100 cells x 106/l. In deciding to initiate treatment with darunavir in such ART-experienced patients, genotypic testing should guide the use of darunavir (see sections 4.2, 4.3, 4.4 and 5.1).600 mg Darunavir Krka, co-administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV-1) infection.Darunavir Krka 600 mg tablets may be used to provide suitable dose regimens (see section 4.2):For the treatment of HIV-1 infection in antiretroviral treatment (ART)-experienced adult patients, including those that have been highly pre-treated.For the treatment of HIV-1 infection in paediatric patients from the age of 3 years and at least 15 kg body weight.In deciding to initiate treatment with darunavir co-administered with low dose ritonavir, careful consideration should be given to the treatment history of the individual patient and the patterns of mutations associated with different agents. Genotypic or phenotypic testing (when available) and treatment history should guide the use of darunavir. |
Authorised | yes | no | no | 2018-01-26 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for PREZISTA
See the table below for patents covering PREZISTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Netherlands | 300425 | ⤷ Try a Trial | |
| Japan | 4776775 | ⤷ Try a Trial | |
| Lithuania | PA2019018 | ⤷ Try a Trial | |
| Spain | 2224096 | ⤷ Try a Trial | |
| Portugal | 2371361 | ⤷ Try a Trial | |
| Cyprus | 1115665 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PREZISTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2371361 | PA2019018 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: PLERIKSAFORAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA, ARBA METALO KOMPLEKSAS; REGISTRATION NO/DATE: EU/1/09/537/001 20090731 |
| 1411918 | 1290023-9 | Sweden | ⤷ Try a Trial | PRODUCT NAME: PLERIXAFOR, VALFRITT I FORMEN AV ETT FARMACEUTISKT GODTAGBART SALT DAERAV; REG. NO/DATE: EU/1/09/534/001 20090804 |
| 0810209 | C300283 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DARUNAVIR, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF ESTER, IN HET BIJZONDER DARUNAVIR ETHANOLAAT; REGISTRATION NO/DATE: EU/1/06/380/001 20070212 |
| 0810209 | 07C0034 | France | ⤷ Try a Trial | PRODUCT NAME: DARUNAVIR; REGISTRATION NO/DATE: EU/1/06/380/001 20070212 |
| 3150586 | LUC00156 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COBICISTAT OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF, DARUNAVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF, IN PARTICULAR DARUNAVIR ETHANOLATE, AND EMTRICITABINE OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF; AUTHORISATION NUMBER AND DATE: EU/1/17/1225 20170925 |
| 2487162 | 284 1-2017 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: KOBICISTAT VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM/DARUNAVIR VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/14/967 20141121 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


