NICOTINE Drug Patent Profile
✉ Email this page to a colleague
When do Nicotine patents expire, and what generic alternatives are available?
Nicotine is a drug marketed by Aveva, Fertin Pharma, Ivax Sub Teva Pharms, L Perrigo Co, P And L, Perrigo R And D, Aurobindo Pharma, Aurobindo Pharma Ltd, Dr Reddys Labs Sa, and Pld Acquisitions. and is included in fifty NDAs.
The generic ingredient in NICOTINE is nicotine polacrilex. There are thirty drug master file entries for this compound. Sixty suppliers are listed for this compound. Additional details are available on the nicotine polacrilex profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Nicotine
A generic version of NICOTINE was approved as nicotine polacrilex by P AND L on March 15th, 1999.
Summary for NICOTINE
| US Patents: | 0 |
| Applicants: | 10 |
| NDAs: | 50 |
| Finished Product Suppliers / Packagers: | 14 |
| Raw Ingredient (Bulk) Api Vendors: | 105 |
| Clinical Trials: | 921 |
| Patent Applications: | 6,093 |
| Formulation / Manufacturing: | see details |
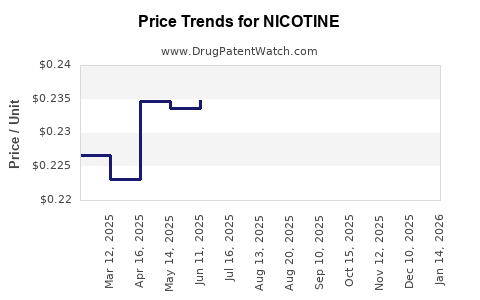
| Drug Prices: | Drug price information for NICOTINE |
| What excipients (inactive ingredients) are in NICOTINE? | NICOTINE excipients list |
| DailyMed Link: | NICOTINE at DailyMed |
Recent Clinical Trials for NICOTINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Alternative Research Initiative (Pvt.) Ltd | Early Phase 1 |
| Foundation for a Smoke-Free World | Early Phase 1 |
| The Netherlands Cancer Institute | Phase 2 |
Pharmacology for NICOTINE
| Drug Class | Cholinergic Nicotinic Agonist |
Medical Subject Heading (MeSH) Categories for NICOTINE
Anatomical Therapeutic Chemical (ATC) Classes for NICOTINE
Paragraph IV (Patent) Challenges for NICOTINE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NICODERM CQ | Transdermal System | nicotine | 7 mg/24 hrs 14 mg/24 hrs 21 mg/24 hrs | 020165 | 1 | 2014-05-30 |


