ERIVEDGE Drug Patent Profile
✉ Email this page to a colleague
When do Erivedge patents expire, and when can generic versions of Erivedge launch?
Erivedge is a drug marketed by Genentech and is included in one NDA. There are three patents protecting this drug.
This drug has fifty-three patent family members in twenty-four countries.
The generic ingredient in ERIVEDGE is vismodegib. One supplier is listed for this compound. Additional details are available on the vismodegib profile page.
DrugPatentWatch® Generic Entry Outlook for Erivedge
Erivedge was eligible for patent challenges on January 30, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 11, 2028. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for ERIVEDGE
| International Patents: | 53 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 127 |
| Clinical Trials: | 46 |
| Patent Applications: | 1,768 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ERIVEDGE |
| What excipients (inactive ingredients) are in ERIVEDGE? | ERIVEDGE excipients list |
| DailyMed Link: | ERIVEDGE at DailyMed |
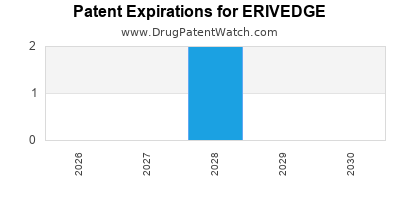

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ERIVEDGE
Generic Entry Date for ERIVEDGE*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ERIVEDGE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| H. Lee Moffitt Cancer Center and Research Institute | Early Phase 1 |
| Genentech, Inc. | Early Phase 1 |
| Ronald Buckanovich | Phase 2 |
Pharmacology for ERIVEDGE
| Drug Class | Hedgehog Pathway Inhibitor |
| Mechanism of Action | Smoothened Receptor Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for ERIVEDGE
US Patents and Regulatory Information for ERIVEDGE
ERIVEDGE is protected by three US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ERIVEDGE is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ERIVEDGE
Pyridyl inhibitors of hedgehog signalling
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pyridyl inhibitors of hedgehog signalling
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF USING VISMODEGIB TO TREAT CANCER IN A MAMMAL
Pyridyl inhibitors of hedgehog signalling
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF USING VISMODEGIB TO TREAT BASAL CELL CARCINOMA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genentech | ERIVEDGE | vismodegib | CAPSULE;ORAL | 203388-001 | Jan 30, 2012 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Genentech | ERIVEDGE | vismodegib | CAPSULE;ORAL | 203388-001 | Jan 30, 2012 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Genentech | ERIVEDGE | vismodegib | CAPSULE;ORAL | 203388-001 | Jan 30, 2012 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ERIVEDGE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Roche Registration GmbH | Erivedge | vismodegib | EMEA/H/C/002602 Erivedge is indicated for the treatment of adult patients with:- symptomatic metastatic basal cell carcinoma- locally advanced basal cell carcinoma inappropriate for surgery or radiotherapy |
Authorised | no | no | no | 2013-07-12 | 2013-04-26 |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ERIVEDGE
When does loss-of-exclusivity occur for ERIVEDGE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 05282722
Estimated Expiration: ⤷ Try a Trial
Patent: 11201229
Estimated Expiration: ⤷ Try a Trial
Patent: 13219216
Estimated Expiration: ⤷ Try a Trial
Patent: 16203958
Estimated Expiration: ⤷ Try a Trial
Patent: 17261491
Estimated Expiration: ⤷ Try a Trial
Patent: 19226273
Estimated Expiration: ⤷ Try a Trial
Patent: 21205133
Estimated Expiration: ⤷ Try a Trial
Patent: 23237067
Estimated Expiration: ⤷ Try a Trial
Austria
Patent: 32768
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0514841
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 79002
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1072755
Estimated Expiration: ⤷ Try a Trial
Patent: 2964294
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 12630
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 89390
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 7262
Estimated Expiration: ⤷ Try a Trial
Patent: 0700538
Estimated Expiration: ⤷ Try a Trial
Patent: 1100604
Estimated Expiration: ⤷ Try a Trial
Patent: 1890903
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 89390
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 300068
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 1673
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 43558
Estimated Expiration: ⤷ Try a Trial
Patent: 08511675
Estimated Expiration: ⤷ Try a Trial
Patent: 12184255
Estimated Expiration: ⤷ Try a Trial
Patent: 14148537
Estimated Expiration: ⤷ Try a Trial
Luxembourg
Patent: 278
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 07002584
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 9385
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 9260
Estimated Expiration: ⤷ Try a Trial
Patent: 071719
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 89390
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 89390
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 89390
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0702537
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1225018
Estimated Expiration: ⤷ Try a Trial
Patent: 1366414
Estimated Expiration: ⤷ Try a Trial
Patent: 070085243
Estimated Expiration: ⤷ Try a Trial
Patent: 120139846
Estimated Expiration: ⤷ Try a Trial
Patent: 130083488
Estimated Expiration: ⤷ Try a Trial
Patent: 140048343
Estimated Expiration: ⤷ Try a Trial
Patent: 150002863
Estimated Expiration: ⤷ Try a Trial
Patent: 150090263
Estimated Expiration: ⤷ Try a Trial
Patent: 160058972
Estimated Expiration: ⤷ Try a Trial
Patent: 170001725
Estimated Expiration: ⤷ Try a Trial
Patent: 180122750
Estimated Expiration: ⤷ Try a Trial
Patent: 190072678
Estimated Expiration: ⤷ Try a Trial
Patent: 200019263
Estimated Expiration: ⤷ Try a Trial
Patent: 200118909
Estimated Expiration: ⤷ Try a Trial
Patent: 210090744
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 77430
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 565
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ERIVEDGE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 2017261491 | Pyridyl Inhibitors of Hedgehog Signalling | ⤷ Try a Trial |
| South Korea | 20150002863 | PYRIDYL INHIBITORS OF HEDGEHOG SIGNALLING | ⤷ Try a Trial |
| South Korea | 20160058972 | 헤지호그 신호전달에 대한 피리딜 억제제 (PYRIDYL INHIBITORS OF HEDGEHOG SIGNALLING) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ERIVEDGE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1789390 | CR 2013 00050 | Denmark | ⤷ Try a Trial | PRODUCT NAME: VISMODEGIB; NAT. REG. NO/DATE: EU71/13/848 20130712; FIRST REG. NO/DATE: CH 62497 20130530 |
| 1789390 | C300614 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: VISMODEGIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF SOLVAAT; REGISTRATION NO/DATE: EU/1/13/848 20130712 |
| 1789390 | 2013C/071 | Belgium | ⤷ Try a Trial | PRODUCT NAME: VISMODEGIB; AUTHORISATION NUMBER AND DATE: EU/1/13/84/001 20130715 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


