CYCLOSPORINE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Cyclosporine, and what generic alternatives are available?
Cyclosporine is a drug marketed by Apotex, Dr Reddys Labs Sa, Ivax Sub Teva Pharms, Sandoz, Strides Pharma, Mylan, Teva Pharms Usa Inc, Hikma, Padagis Us, Abbvie, Pharm Assoc, and Pharmobedient Cnsltg. and is included in sixteen NDAs.
The generic ingredient in CYCLOSPORINE is cyclosporine. There are eighteen drug master file entries for this compound. Eighteen suppliers are listed for this compound. Additional details are available on the cyclosporine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Cyclosporine
A generic version of CYCLOSPORINE was approved as cyclosporine by HIKMA on October 29th, 1999.
Summary for CYCLOSPORINE
| US Patents: | 0 |
| Applicants: | 12 |
| NDAs: | 16 |
| Finished Product Suppliers / Packagers: | 10 |
| Raw Ingredient (Bulk) Api Vendors: | 89 |
| Clinical Trials: | 1,050 |
| Patent Applications: | 7,583 |
| Formulation / Manufacturing: | see details |
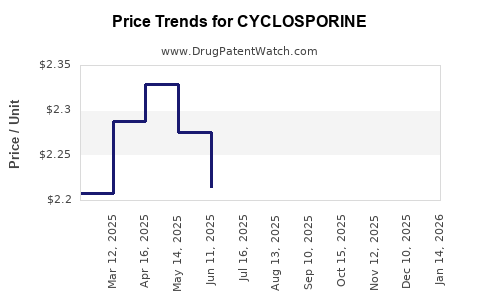
| Drug Prices: | Drug price information for CYCLOSPORINE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for CYCLOSPORINE |
| What excipients (inactive ingredients) are in CYCLOSPORINE? | CYCLOSPORINE excipients list |
| DailyMed Link: | CYCLOSPORINE at DailyMed |
Recent Clinical Trials for CYCLOSPORINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Beijing Mabworks Biotech Co., Ltd. | Phase 3 |
| Aarhus University Hospital, Department of Nephrology | Phase 4 |
| Odense University Hospital, Department of Nephrology | Phase 4 |
Pharmacology for CYCLOSPORINE
| Drug Class | Calcineurin Inhibitor Immunosuppressant |
| Mechanism of Action | Calcineurin Inhibitors Cytochrome P450 3A4 Inhibitors P-Glycoprotein Inhibitors |
Medical Subject Heading (MeSH) Categories for CYCLOSPORINE
Paragraph IV (Patent) Challenges for CYCLOSPORINE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| RESTASIS MULTIDOSE | Ophthalmic Emulsion | cyclosporine | 0.05% | 050790 | 1 | 2014-01-13 |
US Patents and Regulatory Information for CYCLOSPORINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apotex | CYCLOSPORINE | cyclosporine | CAPSULE;ORAL | 065040-001 | May 9, 2002 | AB2 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Teva Pharms Usa Inc | CYCLOSPORINE | cyclosporine | EMULSION;OPHTHALMIC | 203880-001 | Dec 14, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Pharm Assoc | CYCLOSPORINE | cyclosporine | SOLUTION;ORAL | 065167-001 | Jan 5, 2005 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Dr Reddys Labs Sa | CYCLOSPORINE | cyclosporine | CAPSULE;ORAL | 065044-001 | Dec 20, 2000 | AB1 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Apotex | CYCLOSPORINE | cyclosporine | CAPSULE;ORAL | 065040-002 | May 9, 2002 | AB2 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


