ACANYA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Acanya, and what generic alternatives are available?
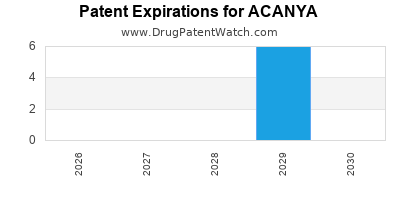
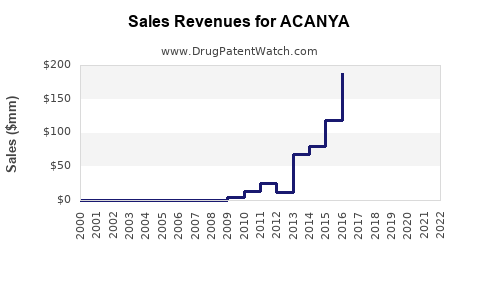
Acanya is a drug marketed by Bausch and is included in one NDA. There are six patents protecting this drug.
This drug has twenty patent family members in fourteen countries.
The generic ingredient in ACANYA is benzoyl peroxide; clindamycin phosphate. There are seventeen drug master file entries for this compound. Fourteen suppliers are listed for this compound. Additional details are available on the benzoyl peroxide; clindamycin phosphate profile page.
DrugPatentWatch® Generic Entry Outlook for Acanya
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for ACANYA
| International Patents: | 20 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 6 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ACANYA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ACANYA |
| What excipients (inactive ingredients) are in ACANYA? | ACANYA excipients list |
| DailyMed Link: | ACANYA at DailyMed |

Recent Clinical Trials for ACANYA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Taro Pharmaceuticals USA | Phase 1 |
| Watson Laboratories, Inc. | Phase 3 |
| Perrigo Company | Phase 3 |
Pharmacology for ACANYA
| Drug Class | Lincosamide Antibacterial |
| Physiological Effect | Decreased Sebaceous Gland Activity |
Anatomical Therapeutic Chemical (ATC) Classes for ACANYA
US Patents and Regulatory Information for ACANYA
ACANYA is protected by six US patents.
Patents protecting ACANYA
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ACANYA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bausch | ACANYA | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-001 | Oct 23, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ACANYA
When does loss-of-exclusivity occur for ACANYA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09255679
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0913326
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 23029
Estimated Expiration: ⤷ Try a Trial
China
Patent: 2056481
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0200450
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 99810
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 77693
Estimated Expiration: ⤷ Try a Trial
Patent: 06272
Estimated Expiration: ⤷ Try a Trial
Patent: 11522820
Estimated Expiration: ⤷ Try a Trial
Patent: 15038093
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 10013152
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 99810
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 93847
Estimated Expiration: ⤷ Try a Trial
Patent: 45087
Estimated Expiration: ⤷ Try a Trial
Patent: 10146038
Estimated Expiration: ⤷ Try a Trial
Patent: 13122395
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1008265
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 110014651
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 73931
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ACANYA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 0626844 | COMPOSITIONS DE CLINDAMYCINE ET DE PEROXYDE DE BENZOYLE POUR LE TRAITEMENT DE L'ACNE (COMPOSITIONS OF CLINDAMYCIN AND BENZOYL PEROXIDE FOR ACNE TREATMENT) | ⤷ Try a Trial |
| Russian Federation | 2645087 | ФАРМАЦЕВТИЧЕСКИЕ СОСТАВЫ ДЛЯ МЕСТНОГО ПРИМЕНЕНИЯ, СОДЕРЖАЩИЕ НИЗКУЮ КОНЦЕНТРАЦИЮ БЕНЗОИЛПЕРОКСИДА В СУСПЕНЗИИ В ВОДЕ И СМЕШИВАЮЩИМСЯ С ВОДОЙ ОРГАНИЧЕСКОМ РАСТВОРИТЕЛЕ (PHARMACEUTICAL FORMULATIONS FOR LOCAL APPLICATION CONTAINING LOW CONCENTRATIONS OF BENZOYL PEROXIDE IN SUSPENSION IN WATER AND WATER-MISCIBLE ORGANIC SOLVENT) | ⤷ Try a Trial |
| Australia | 666169 | ⤷ Try a Trial | |
| Brazil | 9305884 | ⤷ Try a Trial | |
| New Zealand | 249021 | ACIDIC GEL COMPOSITIONS CONTAINING BENZOYL PEROXIDE AND CLINDAMYCIN FOR TREATING ACNE, AND KITS | ⤷ Try a Trial |
| Australia | 2009255679 | Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ACANYA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0186118 | SPC/GB05/029 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: MESOTRIONE (2-(4-METHYLSULPHONYL-2-NITROBENZOYL)-1,3CYCLOHEXANEDIONE); REGISTERED: AU 2726 20001016; UK 0309 OF 2005 20050218 |
| 1458369 | CA 2008 00029 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ADAPALEN, BENZOYLPEROXID |
| 0137963 | 97C0042 | Belgium | ⤷ Try a Trial | PRODUCT NAME: 2-(2-BENZOYL-SUBSTITUE)-1,3-CYCLOHEXANE-DIONES; REGISTRATION NO/DATE: 8452/B 19930121 |
| 0526708 | C300097 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: BOSENTAN, DESGEWENST IN DE VORM VAN EEN ZOUT OF EEN HYDRAAT OF IN DE VORM VAN EEN ESTER VAN DE HYDROXYLGROEP VAN DE 2-HYDROXYETHOXY REST MET EEN ZUUR MET DE FORMULE R5-OH, WAARIN R5 EEN C1-7-ALKANOYL, BENZOYL, OF HETEROCYCLYCARBONYL VOORSTELT; NATL. REGISTRATION NO/DATE: U/1/02/220/001 - 005 20020515; FIRST REGISTRATION: CH IKS 58841 01 - 02 20020228 |
| 1458369 | 122008000041 | Germany | ⤷ Try a Trial | PRODUCT NAME: ADAPALEN IN KOMBINATION MIT BENZOYLPEROXID; NAT. REGISTRATION NO/DATE: 67913.00.00 20080229; FIRST REGISTRATION: DAENEMARK 40440 20071218 |
| 1667986 | 92172 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


