Mallinckrodt Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for MALLINCKRODT INC, and when can generic versions of MALLINCKRODT INC drugs launch?
MALLINCKRODT INC has four approved drugs.
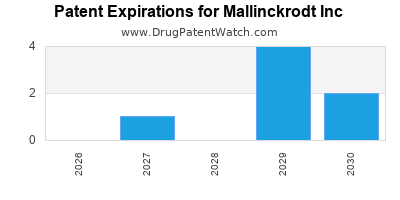
There are twelve US patents protecting MALLINCKRODT INC drugs.
There are thirty-one patent family members on MALLINCKRODT INC drugs in thirteen countries and sixty-seven supplementary protection certificates in eight countries.
Summary for Mallinckrodt Inc
| International Patents: | 31 |
| US Patents: | 12 |
| Tradenames: | 4 |
| Ingredients: | 4 |
| NDAs: | 4 |
| Drug Master File Entries: | 67 |
Drugs and US Patents for Mallinckrodt Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 8,741,885 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mallinckrodt Inc | BAROS | sodium bicarbonate; tartaric acid | GRANULE, EFFERVESCENT;ORAL | 018509-001 | Aug 7, 1985 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 8,394,408 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 8,377,453 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | DISCN | Yes | No | 7,976,870 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Mallinckrodt Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Mallinckrodt Inc | XARTEMIS XR | acetaminophen; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204031-001 | Mar 11, 2014 | 6,488,962 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for MALLINCKRODT INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 7.5 mg/325 mg | ➤ Subscribe | 2014-04-03 |
Premature patent expirations for MALLINCKRODT INC
Expiration due to failure to pay maintenance fee
| Patent Number | Expiration Date |
|---|---|
| ⤷ Try a Trial | ⤷ Try a Trial |
International Patents for Mallinckrodt Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 2654727 | ⤷ Try a Trial |
| Japan | 2014500315 | ⤷ Try a Trial |
| Canada | 2409552 | ⤷ Try a Trial |
| Hong Kong | 1152469 | ⤷ Try a Trial |
| Australia | 2009223061 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Mallinckrodt Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0268956 | SPC/GB98/040 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: RABEPRAZOLE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE SODIUM SALT; REGISTERED: UK 10555/0010 19980508; UK 10555/0008 19980508 |
| 2673237 | LUC00111 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SODIUM ZIRCONIUM CYCLOSILICATE; AUTHORISATION NUMBER AND DATE: EU/1/17/1173 20180326 |
| 2380576 | 20C1048 | France | ⤷ Try a Trial | PRODUCT NAME: SEL DE SODIUM DE L'ACIDE DESOXYCHOLIQUE; NAT. REGISTRATION NO/DATE: NL46299 20180810; FIRST REGISTRATION: IS - IS/1/16/071/01 20160729 |
| 2822954 | 18C1035 | France | ⤷ Try a Trial | PRODUCT NAME: BICTEGRAVIR OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER BICTEGRAVIR DE SODIUM; REGISTRATION NO/DATE: EU/1/18/1289 20180625 |
| 1856135 | LUC00153 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: FOSTAMATINIB OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE FOSTAMATINIB, OU UN HYDRATE, SOLVATE OU N-OXYDE DE FOSTAMATINIB OU LE SEL PHARMACEUTIQUEMENT ACCEPTABLE DE FOSTAMATINIB, EN PARTICULIER FOSTAMATINIB DISODIUM, EVENTUELLEMENT SOUS FORME D'HYDRATE; AUTHORISATION NUMBER AND DATE: EU/1/19/1405 20200113 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.