Gilead Sciences Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GILEAD SCIENCES INC, and what generic alternatives to GILEAD SCIENCES INC drugs are available?
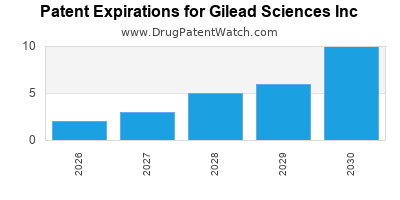
GILEAD SCIENCES INC has twenty-three approved drugs.
There are eighty-two US patents protecting GILEAD SCIENCES INC drugs.
There are two thousand two hundred and twenty-eight patent family members on GILEAD SCIENCES INC drugs in sixty-six countries and three hundred and seventy supplementary protection certificates in nineteen countries.
Summary for Gilead Sciences Inc
| International Patents: | 2228 |
| US Patents: | 82 |
| Tradenames: | 18 |
| Ingredients: | 18 |
| NDAs: | 23 |
| Drug Master File Entries: | 7 |
| Patent Litigation for Gilead Sciences Inc: | See patent lawsuits for Gilead Sciences Inc |
Drugs and US Patents for Gilead Sciences Inc
Expired US Patents for Gilead Sciences Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | STRIBILD | cobicistat; elvitegravir; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 203100-001 | Aug 27, 2012 | 6,703,396*PED | ⤷ Try a Trial |
| Gilead Sciences Inc | VIREAD | tenofovir disoproxil fumarate | TABLET;ORAL | 021356-004 | Jan 18, 2012 | 5,977,089*PED | ⤷ Try a Trial |
| Gilead Sciences Inc | ZYDELIG | idelalisib | TABLET;ORAL | 205858-002 | Jul 23, 2014 | 8,637,533 | ⤷ Try a Trial |
| Gilead Sciences Inc | ODEFSEY | emtricitabine; rilpivirine hydrochloride; tenofovir alafenamide fumarate | TABLET;ORAL | 208351-001 | Mar 1, 2016 | 6,838,464 | ⤷ Try a Trial |
| Gilead Sciences Inc | COMPLERA | emtricitabine; rilpivirine hydrochloride; tenofovir disoproxil fumarate | TABLET;ORAL | 202123-001 | Aug 10, 2011 | 6,703,396*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GILEAD SCIENCES INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2010-01-26 |
| ➤ Subscribe | Tablets | 200 mg/25 mg/300 mg | ➤ Subscribe | 2015-05-20 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2015-12-09 |
| ➤ Subscribe | Tablets | 150 mg, 200 mg, and 250 mg | ➤ Subscribe | 2012-05-17 |
| ➤ Subscribe | Tablets | 150 mg, 150 mg, 200 mg, 300 mg | ➤ Subscribe | 2018-10-04 |
International Patents for Gilead Sciences Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Norway | 2017066 | ⤷ Try a Trial |
| South Africa | 201207800 | ⤷ Try a Trial |
| Lithuania | C3150586 | ⤷ Try a Trial |
| Serbia | 57718 | ⤷ Try a Trial |
| European Patent Office | 1636190 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Gilead Sciences Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2487163 | 2016C/066 | Belgium | ⤷ Try a Trial | PRODUCT NAME: COBICISTAT ET ATAZANAVIR; AUTHORISATION NUMBER AND DATE: EU/1/15/1025 20150715 |
| 1419152 | 132012902054377 | Italy | ⤷ Try a Trial | PRODUCT NAME: EMTRICITABINA/RILPIVIRINA CLORIDRATO/TENOFOVIR DISOPROXIL FUMARATO(EVIPLERA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/11/737/001-002, 20111128 |
| 2822954 | 2018/031 | Ireland | ⤷ Try a Trial | PRODUCT NAME: BICTEGRAVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR BICTEGRAVIR SODIUM.; REGISTRATION NO/DATE: EU/1/18/1289 20180625 |
| 1564210 | CA 2013 00058 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ELVITEGRAVIR I ENHVER FORM SOM ER OMFATTET AF GRUNDPATENTET; REG. NO/DATE: EU/1/13/830/001-002 20130524 |
| 2924034 | SPC/GB19/024 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: DORAVIRINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF IN COMBINATION WITH LAMIVUDINE AND TENOFOVIR DISOPROXIL; REGISTERED: UK EU/1/18/1333/001-002 20181122; UK PLGB 5305/0015 20181122 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.