GLAXOSMITHKLINE Company Profile
✉ Email this page to a colleague
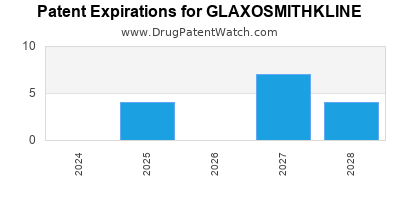
What is the competitive landscape for GLAXOSMITHKLINE, and what generic alternatives to GLAXOSMITHKLINE drugs are available?
GLAXOSMITHKLINE has one hundred and forty-nine approved drugs.
There are thirty-two US patents protecting GLAXOSMITHKLINE drugs.
There are seven hundred and twenty-four patent family members on GLAXOSMITHKLINE drugs in fifty-nine countries and one hundred and eighty-five supplementary protection certificates in twenty countries.
Summary for GLAXOSMITHKLINE
| International Patents: | 724 |
| US Patents: | 32 |
| Tradenames: | 104 |
| Ingredients: | 90 |
| NDAs: | 149 |
| Drug Master File Entries: | 8 |
| Patent Litigation for GLAXOSMITHKLINE: | See patent lawsuits for GLAXOSMITHKLINE |
| PTAB Cases with GLAXOSMITHKLINE as petitioner: | See PTAB cases with GLAXOSMITHKLINE as petitioner |
Drugs and US Patents for GLAXOSMITHKLINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glaxosmithkline Llc | FLOLAN | epoprostenol sodium | INJECTABLE;INJECTION | 020444-001 | Sep 20, 1995 | AP1 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Glaxosmithkline | JESDUVROQ | daprodustat | TABLET;ORAL | 216951-002 | Feb 1, 2023 | RX | Yes | No | 8,815,884 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Glaxosmithkline | THORAZINE | chlorpromazine hydrochloride | CONCENTRATE;ORAL | 009149-032 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Glaxosmithkline | WELLCOVORIN | leucovorin calcium | TABLET;ORAL | 018342-002 | Jul 8, 1983 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Glaxosmithkline | TRELEGY ELLIPTA | fluticasone furoate; umeclidinium bromide; vilanterol trifenatate | POWDER;INHALATION | 209482-002 | Sep 9, 2020 | RX | Yes | Yes | 7,488,827 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GLAXOSMITHKLINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Glaxosmithkline | ARNUITY ELLIPTA | fluticasone furoate | POWDER;INHALATION | 205625-001 | Aug 20, 2014 | 5,873,360 | ⤷ Try a Trial |
| Glaxosmithkline | NIX | permethrin | LOTION;TOPICAL | 019435-001 | Mar 31, 1986 | 4,024,163 | ⤷ Try a Trial |
| Glaxosmithkline | MALARONE PEDIATRIC | atovaquone; proguanil hydrochloride | TABLET;ORAL | 021078-002 | Jul 14, 2000 | 5,998,449*PED | ⤷ Try a Trial |
| Glaxosmithkline | WELLBUTRIN | bupropion hydrochloride | TABLET;ORAL | 018644-003 | Dec 30, 1985 | 3,885,046 | ⤷ Try a Trial |
| Glaxosmithkline Llc | REQUIP XL | ropinirole hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 022008-002 | Jun 13, 2008 | 5,422,123 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GLAXOSMITHKLINE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 3 mg | ➤ Subscribe | 2009-01-08 |
| ➤ Subscribe | Injection | 6 mg/0.5 mL, 0.5 mL vials | ➤ Subscribe | 2004-10-25 |
| ➤ Subscribe | Tablets | 0.25 mg, 0.5 mg, 1 mg and 2 mg | ➤ Subscribe | 2004-12-22 |
| ➤ Subscribe | Tablets | 100 mg | ➤ Subscribe | 2007-10-31 |
| ➤ Subscribe | Tablets | 62.5 mg/25 mg | ➤ Subscribe | 2010-09-14 |
| ➤ Subscribe | Extended-release Capsules | 225 mg and 425 mg | ➤ Subscribe | 2006-10-11 |
| ➤ Subscribe | Extended-release Tablets | 25 mg, 50 mg, 100 mg, 200 mg, 250 mg, and 300 mg | ➤ Subscribe | 2014-02-12 |
| ➤ Subscribe | Extended-release Tablets | 8 mg | ➤ Subscribe | 2008-11-03 |
| ➤ Subscribe | Extended-release Tablets | 12 mg | ➤ Subscribe | 2009-02-05 |
| ➤ Subscribe | Extended-release Tablets | 6 mg | ➤ Subscribe | 2009-07-22 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2007-10-30 |
| ➤ Subscribe | Orally Disintegrating Tablets | 25 mg, 50 mg, 100 mg, and 200 mg | ➤ Subscribe | 2009-12-21 |
| ➤ Subscribe | Injection | 6 mg/0.5 mL, 0.5 mL (prefilled syringes) | ➤ Subscribe | 2006-05-09 |
| ➤ Subscribe | Tablets | 3 mg, 4 mg and 5 mg | ➤ Subscribe | 2005-02-04 |
| ➤ Subscribe | Tablets | 250 mg/100 mg | ➤ Subscribe | 2009-04-03 |
| ➤ Subscribe | Extended-release Capsules | 325 mg | ➤ Subscribe | 2006-11-07 |
| ➤ Subscribe | Oral Suspension | 750 mg/5 mL | ➤ Subscribe | 2009-10-20 |
| ➤ Subscribe | Extended-release Tablets | 4 mg | ➤ Subscribe | 2008-10-31 |
| ➤ Subscribe | Extended-release Tablets | 3 mg | ➤ Subscribe | 2009-01-08 |
| ➤ Subscribe | Extended-release Tablets | 2 mg | ➤ Subscribe | 2008-10-14 |
International Patents for GLAXOSMITHKLINE Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Germany | 60326590 | ⤷ Try a Trial |
| Australia | 2004220321 | ⤷ Try a Trial |
| Hungary | S1400053 | ⤷ Try a Trial |
| Cyprus | 1123604 | ⤷ Try a Trial |
| Peru | 20170915 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for GLAXOSMITHKLINE Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1425001 | 174 50004-2014 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: VILANTEROLTRIFENATAT; REGISTRATION NO/DATE: EU/1/13/886/001 - EU/1/13/886/006 20131113 |
| 1740177 | 92565 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: BROMURE D'UMECLIDINUM; FIRST REGISTRATION DATE: 20140428 |
| 2109608 | 667 | Finland | ⤷ Try a Trial | |
| 0656775 | 28/2000 | Austria | ⤷ Try a Trial | PRODUCT NAME: BUPROPION HYDROCHLORID; NAT. REGISTRATION NO/DATE: 1-23680 20000616; FIRST REGISTRATION: NL 24160 19991201 |
| 1110543 | SPC/GB08/005 | United Kingdom | ⤷ Try a Trial | SUPPLEMENTARY PROTECTION CERTIFICATE NO SPC/GB08/005 GRANTED TO MERCK SHARP + DOHME CORP. IN RESPECT OF THE PRODUCT DESLORATADINE OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, IN COMBINATION WITH PSEUDOEPHEDRINE OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT OR ESTER, THE GRANT OF WHICH WAS ADVERTISED IN JOURNAL NO 6322 DATED 21 JULY 2010 HAS HAD ITS MAXIMUM PERIOD OF DURATION CORRECTED, SUBJECT TO THE PAYMENT OF THE PRESCRIBED FEES IT WILL EXPIRE ON 31 JULY 2022. |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.