ASTELLAS Company Profile
✉ Email this page to a colleague
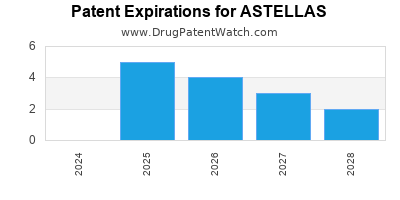
What is the competitive landscape for ASTELLAS, and when can generic versions of ASTELLAS drugs launch?
ASTELLAS has thirty-seven approved drugs.
There are twenty-five US patents protecting ASTELLAS drugs. There is one tentative approval on ASTELLAS drugs.
There are four hundred and thirty-three patent family members on ASTELLAS drugs in forty-two countries and sixty-five supplementary protection certificates in nineteen countries.
Summary for ASTELLAS
| International Patents: | 433 |
| US Patents: | 25 |
| Tradenames: | 21 |
| Ingredients: | 16 |
| NDAs: | 37 |
| Patent Litigation for ASTELLAS: | See patent lawsuits for ASTELLAS |
Drugs and US Patents for ASTELLAS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astellas | PROGRAF | tacrolimus | FOR SUSPENSION;ORAL | 210115-001 | May 24, 2018 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astellas | ADENOCARD | adenosine | INJECTABLE;INJECTION | 019937-002 | Oct 30, 1989 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astellas | CRESEMBA | isavuconazonium sulfate | CAPSULE;ORAL | 207500-001 | Mar 6, 2015 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astellas | CRESEMBA | isavuconazonium sulfate | POWDER;INTRAVENOUS | 207501-001 | Mar 6, 2015 | RX | Yes | Yes | 6,812,238*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astellas | XOSPATA | gilteritinib fumarate | TABLET;ORAL | 211349-001 | Nov 28, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astellas | VESICARE | solifenacin succinate | TABLET;ORAL | 021518-001 | Nov 19, 2004 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ASTELLAS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astellas | LEXISCAN | regadenoson | SOLUTION;INTRAVENOUS | 022161-001 | Apr 10, 2008 | 7,655,637 | ⤷ Try a Trial |
| Astellas | MYCAMINE | micafungin sodium | INJECTABLE;INTRAVENOUS | 021506-002 | Mar 16, 2005 | 6,265,536 | ⤷ Try a Trial |
| Astellas | ASTAGRAF XL | tacrolimus | CAPSULE, EXTENDED RELEASE;ORAL | 204096-003 | Jul 19, 2013 | 6,440,458 | ⤷ Try a Trial |
| Astellas | ASTAGRAF XL | tacrolimus | CAPSULE, EXTENDED RELEASE;ORAL | 204096-002 | Jul 19, 2013 | 6,884,433 | ⤷ Try a Trial |
| Astellas | ASTAGRAF XL | tacrolimus | CAPSULE, EXTENDED RELEASE;ORAL | 204096-001 | Jul 19, 2013 | 6,884,433 | ⤷ Try a Trial |
| Astellas | LEXISCAN | regadenoson | SOLUTION;INTRAVENOUS | 022161-001 | Apr 10, 2008 | 7,144,872 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ASTELLAS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Capsules | 0.5 mg, 1 mg, and 5 mg | ➤ Subscribe | 2013-11-15 |
| ➤ Subscribe | For Injection | 50 mg/vial and 100 mg/vial | ➤ Subscribe | 2014-06-16 |
| ➤ Subscribe | Injection | 3 mg/mL, 20 mL and 30 mL vials | ➤ Subscribe | 2005-04-16 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2009-04-08 |
| ➤ Subscribe | Capsules | 40 mg | ➤ Subscribe | 2016-08-31 |
| ➤ Subscribe | Injection | 3 mg/mL, 20 mL and 30 mL vials | ➤ Subscribe | 2005-04-18 |
| ➤ Subscribe | Injection | 0.08 mg/mL, 5 mL vial | ➤ Subscribe | 2012-04-10 |
International Patents for ASTELLAS Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Russian Federation | 2449993 | ⤷ Try a Trial |
| Japan | 6404217 | ⤷ Try a Trial |
| Australia | 2011255951 | ⤷ Try a Trial |
| Brazil | 112015024907 | ⤷ Try a Trial |
| Singapore | 11201510315T | ⤷ Try a Trial |
| Mexico | 2015003140 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for ASTELLAS Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2428508 | SPC/GB20/007 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: GILTERITINIB OR A SALT THEREOF; REGISTERED: UK EU/1/19/1399(FOR NI) 20191028; UK PLGB00166/0425 20191028 |
| 2428508 | PA2020002 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: GILTERITINIBAS; REGISTRATION NO/DATE: EU/1/19/1399 20191024 |
| 1893196 | 122013000155 | Germany | ⤷ Try a Trial | PRODUCT NAME: ENZALUTAMID ODER EIN PHARMAZEUTISCH VERTRAEGLICHES SALZ HIERVON; REGISTRATION NO/DATE: EU/1/13/846 20130621 |
| 2428508 | CA 2020 00006 | Denmark | ⤷ Try a Trial | PRODUCT NAME: GILTERITINIB ELLER ET SALT DERAF; REG. NO/DATE: EU/1/19/1399 20191028 |
| 1280795 | CR 2016 00002 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ISAVUCONAZOLE AS ISAVUCONAZONIUM SULFATE OR AS AN ISAVUCONAZONIUM SALT WITH ANY OTHER PHARMACEUTICALLY ACCEPTABLE ANION, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE, OR SOLVATE; REG. NO/DATE: EU/1/15/1036 20151019 |
| 1893196 | C 2013 041 | Romania | ⤷ Try a Trial | PRODUCT NAME: ENZALUTAMIDA SAU O SARE ACCEPTABILA FARMACEUTIC AACESTEIA; NATIONAL AUTHORISATION NUMBER: EU/1/13/846; DATE OF NATIONAL AUTHORISATION: 20130621; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/846; DATE OF FIRST AUTHORISATION IN EEA: 20130621 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.